Page 959 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 959
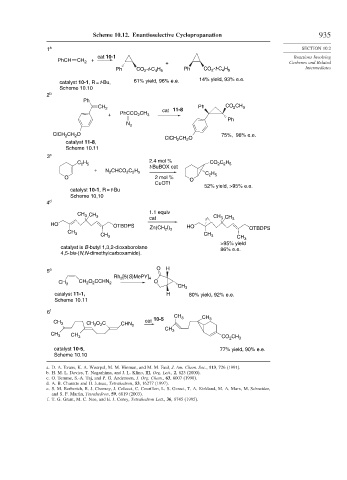
Scheme 10.12. Enantioselective Cyclopropanation 935
1 a SECTION 10.2
cat 10-1 Reactions Involving
PhCH CH 2 +
+ Carbenes and Related
Ph CO 2 -t-C 4 H 9 Ph CO 2 -t-C 4 H 9 Intermediates
catalyst 10-1, R = t-Bu, 61% yield, 96% e.e. 14% yield, 93% e.e.
Scheme 10.10
2 b
Ph
CH 2 Ph CO 2 CH 3
cat 11-8
+ PhCCO 2 CH 3
Ph
N 2
ClCH 2 CH 2 O 75%, 98% e.e.
ClCH 2 CH 2 O
catalyst 11-8,
Scheme 10.11
3 c
2.4 mol %
C 2 H 5 CO 2 C 2 H 5
t-BuBOX cat
+ N 2 CHCO 2 C 2 H 5
C 2 H 5
O 2 mol % O
CuOTf
52% yield, >95% e.e.
catalyst 10-1, R = t-Bu
Scheme 10,10
4 d
1.1 equiv
CH 3 CH 3
cat CH 3 CH 3
HO OTBDPS HO
Zn(CH 2 I) 2 OTBDPS
CH 3
CH 3 CH 3
CH 3
>95% yield
catalyst is B-butyl 1,3,2-dioxaborolane 86% e.e.
4,5-bis-(N,N-dimethylcarboxamide).
5 e O H
Rh 2 [5(S)MePY] 4
CH 2 O 2 CCHN 2 O
CH 3
CH 3
catalyst 11-1, H 80% yield, 92% e.e.
Scheme 10.11
6 f
cat 10-5 CH 3 CH 3
CH 3 CH 3 O 2 C CHN 2
CH 3
CH 3
CH 3
CO 2 CH 3
catalyst 10-5, 77% yield, 90% e.e.
Scheme 10.10
a. D. A. Evans, K. A. Woerpel, M. M. Hinman, and M. M. Faul, J. Am. Chem. Soc., 113, 726 (1991).
b. H. M. L. Davies, T. Nagashima, and J. L. Klino, III, Org. Lett., 2, 823 (2000).
c. O. Temme, S.-A. Taj, and P. G. Andersson, J. Org. Chem., 63, 6007 (1998).
d. A. B. Charette and H. Juteau, Tetrahedron, 53, 16277 (1997).
e. S. M. Berberich, R. J. Cherney, J. Colucci, C. Courillon, L. S. Geraci, T. A. Kirkland, M. A. Marx, M. Schneider,
and S. F. Martin, Tetrahedron, 59, 6819 (2003).
f. T. G. Grant, M. C. Noe, and E. J. Corey, Tetrahedron Lett., 36, 8745 (1995).