Page 962 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 962
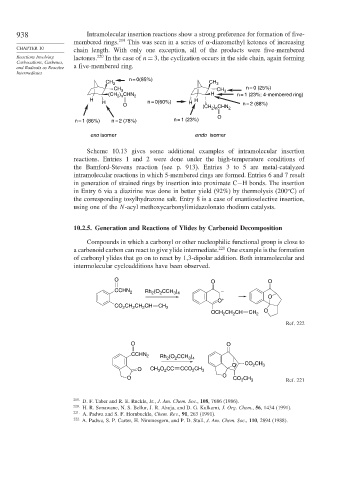
938 Intramolecular insertion reactions show a strong preference for formation of five-
membered rings. 219 This was seen in a series of -diazomethyl ketones of increasing
CHAPTER 10 chain length. With only one exception, all of the products were five-membered
Reactions Involving lactones. 220 In the case of n = 3, the cyclization occurs in the side chain, again forming
Carbocations, Carbenes,
and Radicals as Reactive a five-membered ring.
Intermediates
n = 0(85%)
CH 3 CH 3
CH 3 CH n = 0 (25%)
(CH ) CHN 2 H 3 n = 1 (23%; 4-membered ring)
2 n
H H
H n = 0(60%) H n = 2 (88%)
O (CH ) CHN 2
2 n
O
n = 1 (86%) n = 2 (78%) n = 1 (23%)
exo isomer endo isomer
Scheme 10.13 gives some additional examples of intramolecular insertion
reactions. Entries 1 and 2 were done under the high-temperature conditions of
the Bamford-Stevens reaction (see p. 913). Entries 3 to 5 are metal-catalyzed
intramolecular reactions in which 5-membered rings are formed. Entries 6 and 7 result
in generation of strained rings by insertion into proximate C−H bonds. The insertion
in Entry 6 via a diazirine was done in better yield (92%) by thermolysis (200 C) of
the corresponding tosylhydrazone salt. Entry 8 is a case of enantioselective insertion,
using one of the N-acyl methoxycarbonylimidazolonato rhodium catalysts.
10.2.5. Generation and Reactions of Ylides by Carbenoid Decomposition
Compounds in which a carbonyl or other nucleophilic functional group is close to
a carbenoid carbon can react to give ylide intermediate. 221 One example is the formation
of carbonyl ylides that go on to react by 1,3-dipolar addition. Both intramolecular and
intermolecular cycloadditions have been observed.
O O O
CCHN 2 Rh 2 (O CCH ) –
2
3 4
O
O +
CO CH CH CH CH 2
2
2
2
CH CH CH O
OCH 2 2 2
Ref. 222
O O
CCHN 2 Rh (O CCH )
2
3 4
2
2
O CO CH 3
O CH O CC CCO CH 3
2
2
3
O
O CO CH 3 Ref. 221
2
219
D. F. Taber and R. E. Ruckle, Jr., J. Am. Chem. Soc., 108, 7686 (1986).
220
H. R. Sonawane, N. S. Bellur, J. R. Ahuja, and D. G. Kulkarni, J. Org. Chem., 56, 1434 (1991).
221 A. Padwa and S. F. Hornbuckle, Chem. Rev., 91, 263 (1991).
222
A. Padwa, S. P. Carter, H. Nimmesgern, and P. D. Stull, J. Am. Chem. Soc., 110, 2894 (1988).