Page 967 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 967
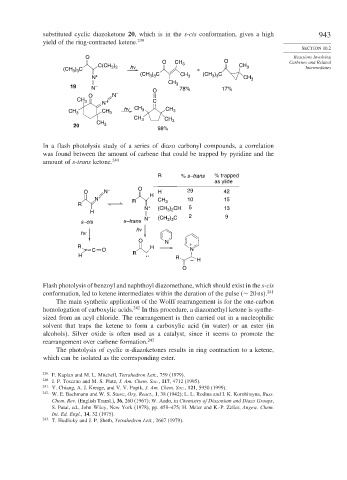
substituted cyclic diazoketone 20, which is in the s-cis conformation, gives a high 943
yield of the ring-contracted ketene. 239
SECTION 10.2
O Reactions Involving
O CH 3 O Carbenes and Related
C(CH ) h ν CH 3
3 3
(CH ) C + Intermediates
3 3
3 3
3 3
N + (CH ) C CH 3 (CH ) C CH 3
CH
19 N – O 3 78% 17%
O N –
CH 3 + C
N
h ν CH CH
CH 3 CH 3 3 3
CH 3
CH CH 3
20 3
98%
In a flash photolysis study of a series of diazo carbonyl compounds, a correlation
was found between the amount of carbene that could be trapped by pyridine and the
amount of s-trans ketone. 240
R % s–trans % trapped
as ylide
O N – O H H 29 42
N + R CH 10 15
R 3
N + (CH ) CH 5 13
H 3 2 2
) C
s–cis s–trans N – (CH 3 3 9
h ν
h ν
O N
R H +
C O N
H R : R
– H
O
Flash photolysis of benzoyl and naphthoyl diazomethane, which should exist in the s-cis
conformation, led to ketene intermediates within the duration of the pulse (∼ 20ns). 241
The main synthetic application of the Wolff rearrangement is for the one-carbon
homologation of carboxylic acids. 242 In this procedure, a diazomethyl ketone is synthe-
sized from an acyl chloride. The rearrangement is then carried out in a nucleophilic
solvent that traps the ketene to form a carboxylic acid (in water) or an ester (in
alcohols). Silver oxide is often used as a catalyst, since it seems to promote the
rearrangement over carbene formation. 243
The photolysis of cyclic -diazoketones results in ring contraction to a ketene,
which can be isolated as the corresponding ester.
239 F. Kaplan and M. L. Mitchell, Tetrahedron Lett., 759 (1979).
240 J. P. Toscano and M. S. Platz, J. Am. Chem. Soc., 117, 4712 (1995).
241
Y. Chiang, A. J. Kresge, and V. V. Popik, J. Am. Chem. Soc., 121, 5930 (1999).
242 W. E. Bachmann and W. S. Stuve, Org. React., 1, 38 (1942); L. L. Rodina and I. K. Korobitsyna, Russ.
Chem. Rev. (English Transl.), 36, 260 (1967); W. Ando, in Chemistry of Diazonium and Diazo Groups,
S. Patai, ed., John Wiley, New York (1978), pp. 458–475; H. Meier and K.-P. Zeller, Angew. Chem.
Int. Ed. Engl., 14, 32 (1975).
243
T. Hudlicky and J. P. Sheth, Tetrahedron Lett., 2667 (1979).