Page 250 - Advanced Thermodynamics for Engineers, Second Edition
P. 250
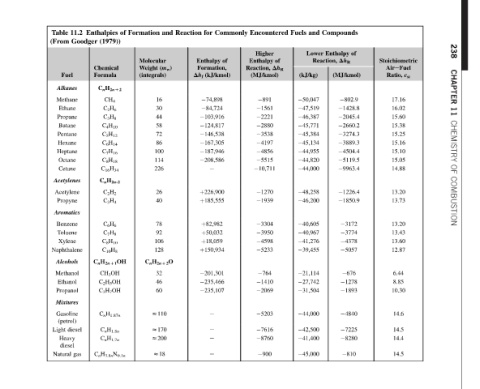
238 CHAPTER 11 CHEMISTRY OF COMBUSTION
Stoichiometric AireFuel ε st Ratio, 17.16 16.02 15.60 15.38 15.25 15.16 15.10 15.05 14.88 13.20 13.73 13.20 13.43 13.60 12.87 6.44 8.85 10.30 14.6 14.5 14.4 14.5
Compounds of Enthalpy Dh R (MJ/kmol) 802.9 1428.8 2045.4 2660.2 3274.3 3889.3 4504.4 5119.5 9963.4 1226.4 1850.9 3172 3774 4378 5057 676 1278 1893 4840 7225 8280 810
and Lower Reaction, (kJ/kg) 50,047 47,519 46,387 45,771 45,384 45,134 44,955 44,820 44,000 48,258 46,200 40,605 40,967 41,276 39,455 21,114 27,742 31,504 44,000 42,500 41,400 45,000
Fuels
Encountered Higher of Enthalpy Dh R Reaction, (MJ/kmol) 891 1561 2221 2880 3538 4197 4856 5515 10,711 1270 1939 3304 3950 4598 5233 764 1410 2069 5203 7616 8760 900
Commonly of
for Enthalpy Formation, (kJ/kmol) 74,898 84,724 103,916 124,817 146,538 167,305 187,946 208,586 e þ226,900 þ185,555 þ82,982 þ50,032 þ18,059 þ150,934 201,301 235,466 235,107 e e e e
Reaction Dh f
and (m w ) 16 30 44 58 72 86 100 114 226 26 40 78 92 106 128 C n H 2nD2 O 32 46 60 z110 z170 z200 z18
Formation Molecular Weight (integrals)
of
Enthalpies (1979)) Chemical Formula C n H 2nD2 CH 4 C 2 H 6 C 3 H 8 C 4 H 10 C 5 H 12 C 6 H 14 C 7 H 16 C 8 H 18 C 16 H 34 C n H 2n-2 C 2 H 2 C 3 H 4 C 6 H 6 C 7 H 8 C 8 H 10 C 10 H 8 C n H 2nD1 OH CH 3 OH C 2 H 5 OH C 3 H 7 OH C n H 1.87n C n H 1.8n C n H 1.7n C n H 3.8n N 0.1n
11.2 Goodger diesel gas
Table (From Fuel Alkanes Methane Ethane Propane Butane Pentane Hexane Heptane Octane Cetane Acetylenes Acetylene Propyne Aromatics Benzene Toluene Xylene Naphthalene Alcohols Methanol Ethanol Propanol Mixtures Gasoline (petrol) Light Heavy diesel Natural