Page 251 - Advanced Thermodynamics for Engineers, Second Edition
P. 251
11.2 ENERGY OF FORMATION 239
also indicates that the energy of formation of the fuel (DH f ) is the net energy required for the
process, i.e.
X X
DH f ¼ DH a DHðX YÞ (11.1)
R
In reality the enthalpy of formation is more complex than given above because energy can be stored
in molecules in a number of ways, including resonance energies (in the benzene ring structure) and
changes of phase (latent heats). A more general representation of the enthalpy of formation is
X X X X
DH f ¼ DH a DHðX YÞ DH res DH latent (11.2)
Example
Evaluate the enthalpy of formation of CO 2 and H 2 O from the atomisation and dissociation energies
listed in Table 11.1.
Solution
Carbon dioxide (CO 2 )
Carbon dioxide is formed from carbon and oxygen in the reaction.
C graphite þ O 2 ðgÞ/CO 2 ðgÞ; (11.3)
where the (g) indicates that the element or compound is in the gaseous (vapour) phase, and ([) will be
used to indicate that the element or compound is in the liquid phase.
The reaction in Eqn (11.3) is achieved by atomisation of the individual carbon (graphite) molecules
and the oxygen molecules, with subsequent recombination to form carbon dioxide. Effectively the
reactant molecules, which are in a metastable state, are activated above a certain energy to produce
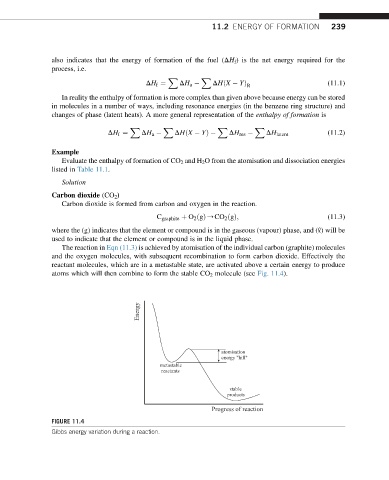
atoms which will then combine to form the stable CO 2 molecule (see Fig. 11.4).
Energy
atomisation
energy "hill"
metastable
reactants
stable
products
Progress of reaction
FIGURE 11.4
Gibbs energy variation during a reaction.