Page 57 - Advanced Thermodynamics for Engineers, Second Edition
P. 57
42 CHAPTER 3 ENGINE CYCLES AND THEIR EFFICIENCIES
(a) (b) 5
Temperature, T Incremental Temperature, T T 5 Incremental
cycles
cycles
Q in Q in
2 3 3 4
T 2 T 3
b c
2 a
1 6' 6
T 1 4 T 1 1
Q out Q out
Saturated Saturated Saturated Saturated
liquid line vapour line liquid line vapour line
Specific entropy, s Specific entropy, s
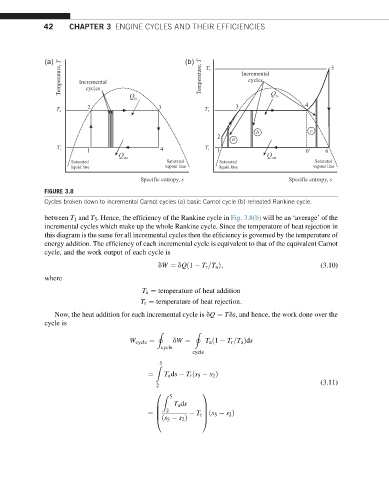
FIGURE 3.8
Cycles broken down to incremental Carnot cycles (a) basic Carnot cycle (b) reheated Rankine cycle.
between T 1 and T 5 . Hence, the efficiency of the Rankine cycle in Fig. 3.8(b) will be an ‘average’ of the
incremental cycles which make up the whole Rankine cycle. Since the temperature of heat rejection in
this diagram is the same for all incremental cycles then the efficiency is governed by the temperature of
energy addition. The efficiency of each incremental cycle is equivalent to that of the equivalent Carnot
cycle, and the work output of each cycle is
dW ¼ dQð1 T r =T a Þ; (3.10)
where
T a ¼ temperature of heat addition
T r ¼ temperature of heat rejection:
Now, the heat addition for each incremental cycle is dQ ¼ Tds, and hence, the work done over the
cycle is
I I
T a ð1 T r =T a Þds
W cycle ¼ dW ¼
cycle
cycle
Z 5
¼ T a ds T r ðs 5 s 2 Þ
(3.11)
2
5
0 1
Z
T a ds
B C
B 2 C
¼ B T rCðs 5 s 2 Þ
A
@ðs 5 s 2 Þ