Page 56 - Advanced Thermodynamics for Engineers, Second Edition
P. 56
3.1 HEAT ENGINES 41
(a) (b) T 5
Temperature, T Additional Temperature, T Additional
work
work
T 3 Q in 4 T 3 Q in 4
W T
W T
2 W P 2 W P
T 6 T
1 1 6' 6
Q out Q out
Saturated Saturated Saturated Saturated
liquid line vapour line liquid line vapour line
Specific entropy, s Specific entropy, s
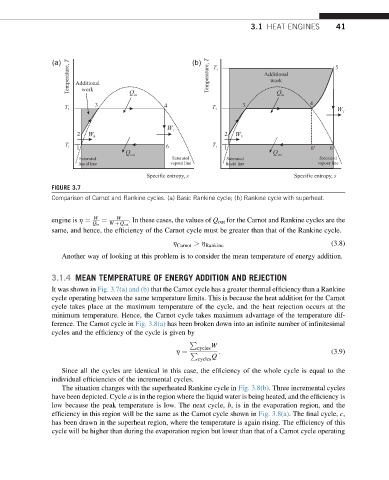
FIGURE 3.7
Comparison of Carnot and Rankine cycles. (a) Basic Rankine cycle; (b) Rankine cycle with superheat.
W W
engine is h ¼ ¼ . In these cases, the values of Q out for the Carnot and Rankine cycles are the
Q in W þ Q out
same, and hence, the efficiency of the Carnot cycle must be greater than that of the Rankine cycle.
h Carnot > h Rankine (3.8)
Another way of looking at this problem is to consider the mean temperature of energy addition.
3.1.4 MEAN TEMPERATURE OF ENERGY ADDITION AND REJECTION
It was shown in Fig. 3.7(a) and (b) that the Carnot cycle has a greater thermal efficiency than a Rankine
cycle operating between the same temperature limits. This is because the heat addition for the Carnot
cycle takes place at the maximum temperature of the cycle, and the heat rejection occurs at the
minimum temperature. Hence, the Carnot cycle takes maximum advantage of the temperature dif-
ference. The Carnot cycle in Fig. 3.8(a) has been broken down into an infinite number of infinitesimal
cycles and the efficiency of the cycle is given by
P
W
cycles
h ¼ P : (3.9)
cycles Q
Since all the cycles are identical in this case, the efficiency of the whole cycle is equal to the
individual efficiencies of the incremental cycles.
The situation changes with the superheated Rankine cycle in Fig. 3.8(b). Three incremental cycles
have been depicted. Cycle a is in the region where the liquid water is being heated, and the efficiency is
low because the peak temperature is low. The next cycle, b, is in the evaporation region, and the
efficiency in this region will be the same as the Carnot cycle shown in Fig. 3.8(a). The final cycle, c,
has been drawn in the superheat region, where the temperature is again rising. The efficiency of this
cycle will be higher than during the evaporation region but lower than that of a Carnot cycle operating