Page 108 - Advanced thermodynamics for engineers
P. 108
4.12 PROBLEMS 93
5 (h - h )/c T p/p = 100
4
Dimensionless steady flow exergy 3 2 1 0 p/p = 0.01
p/p = 10
p/p = 1
p/p = 0.1
3
4
-1
-2 1 2 Non-dimensional temperature (T/T ) 5
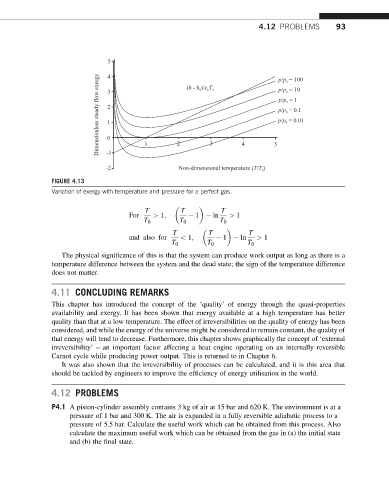
FIGURE 4.13
Variation of exergy with temperature and pressure for a perfect gas.
T T T
For > 1; 1 ln > 1
T 0 T 0 T 0
T T T
and also for < 1; 1 ln > 1
T 0 T 0 T 0
The physical significance of this is that the system can produce work output as long as there is a
temperature difference between the system and the dead state; the sign of the temperature difference
does not matter.
4.11 CONCLUDING REMARKS
This chapter has introduced the concept of the ‘quality’ of energy through the quasi-properties
availability and exergy. It has been shown that energy available at a high temperature has better
quality than that at a low temperature. The effect of irreversibilities on the quality of energy has been
considered, and while the energy of the universe might be considered to remain constant, the quality of
that energy will tend to decrease. Furthermore, this chapter shows graphically the concept of ‘external
irreversibility’ – an important factor affecting a heat engine operating on an internally reversible
Carnot cycle while producing power output. This is returned to in Chapter 6.
It was also shown that the irreversibility of processes can be calculated, and it is this area that
should be tackled by engineers to improve the efficiency of energy utilisation in the world.
4.12 PROBLEMS
P4.1 A piston-cylinder assembly contains 3 kg of air at 15 bar and 620 K. The environment is at a
pressure of 1 bar and 300 K. The air is expanded in a fully reversible adiabatic process to a
pressure of 5.5 bar. Calculate the useful work which can be obtained from this process. Also
calculate the maximum useful work which can be obtained from the gas in (a) the initial state
and (b) the final state.