Page 336 - Advanced thermodynamics for engineers
P. 336
15.2 THERMODYNAMICS OF COMBUSTION 325
Gibbs energy Metastable
equilibrium
Activation
energy
Reactants
Stable
equilibrium
Products
State
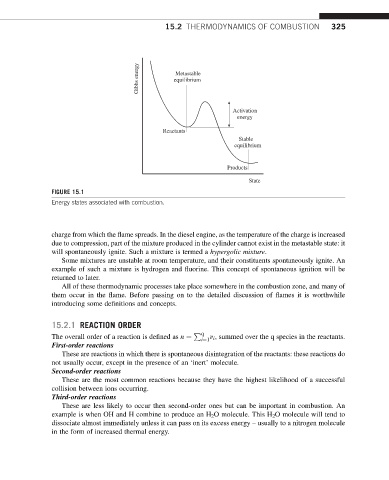
FIGURE 15.1
Energy states associated with combustion.
charge from which the flame spreads. In the diesel engine, as the temperature of the charge is increased
due to compression, part of the mixture produced in the cylinder cannot exist in the metastable state: it
will spontaneously ignite. Such a mixture is termed a hypergolic mixture.
Some mixtures are unstable at room temperature, and their constituents spontaneously ignite. An
example of such a mixture is hydrogen and fluorine. This concept of spontaneous ignition will be
returned to later.
All of these thermodynamic processes take place somewhere in the combustion zone, and many of
them occur in the flame. Before passing on to the detailed discussion of flames it is worthwhile
introducing some definitions and concepts.
15.2.1 REACTION ORDER
q
P
n
The overall order of a reaction is defined as n ¼ i¼1 i , summed over the q species in the reactants.
First-order reactions
These are reactions in which there is spontaneous disintegration of the reactants: these reactions do
not usually occur, except in the presence of an ‘inert’ molecule.
Second-order reactions
These are the most common reactions because they have the highest likelihood of a successful
collision between ions occurring.
Third-order reactions
These are less likely to occur then second-order ones but can be important in combustion. An
example is when OH and H combine to produce an H 2 O molecule. This H 2 O molecule will tend to
dissociate almost immediately unless it can pass on its excess energy – usually to a nitrogen molecule
in the form of increased thermal energy.