Page 37 - Advanced thermodynamics for engineers
P. 37
20 CHAPTER 2 THE SECOND LAW AND EQUILIBRIUM
(a) (b)
(c) (d)
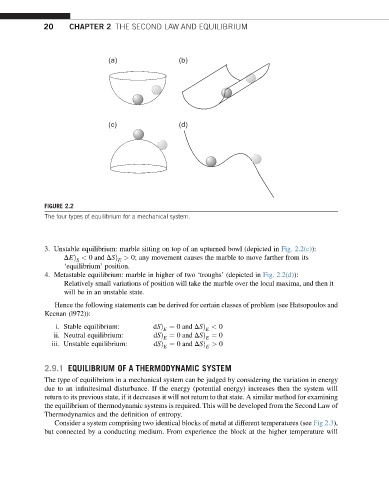
FIGURE 2.2
The four types of equilibrium for a mechanical system.
3. Unstable equilibrium: marble sitting on top of an upturned bowl (depicted in Fig. 2.2(c)):
DEÞ < 0 and DSÞ > 0; any movement causes the marble to move farther from its
S E
‘equilibrium’ position.
4. Metastable equilibrium: marble in higher of two ‘troughs’ (depicted in Fig. 2.2(d)):
Relatively small variations of position will take the marble over the local maxima, and then it
will be in an unstable state.
Hence the following statements can be derived for certain classes of problem (see Hatsopoulos and
Keenan (l972)):
i. Stable equilibrium: dSÞ ¼ 0 and DSÞ < 0
E E
ii. Neutral equilibrium: dSÞ ¼ 0 and DSÞ ¼ 0
E E
iii. Unstable equilibrium: dSÞ ¼ 0 and DSÞ > 0
E E
2.9.1 EQUILIBRIUM OF A THERMODYNAMIC SYSTEM
The type of equilibrium in a mechanical system can be judged by considering the variation in energy
due to an infinitesimal disturbance. If the energy (potential energy) increases then the system will
return to its previous state, if it decreases it will not return to that state. A similar method for examining
the equilibrium of thermodynamic systems is required. This will be developed from the Second Law of
Thermodynamics and the definition of entropy.
Consider a system comprising two identical blocks of metal at different temperatures (see Fig 2.3),
but connected by a conducting medium. From experience the block at the higher temperature will