Page 40 - Advanced thermodynamics for engineers
P. 40
2.10 HELMHOLTZ ENERGY (HELMHOLTZ FUNCTION) 23
In the previous section the criteria for equilibrium were discussed and these were derived in terms
of DS) E . The variation of entropy is not always easy to visualise, and it would be more useful if the
criteria could be derived in a more tangible form related to other properties of the system under
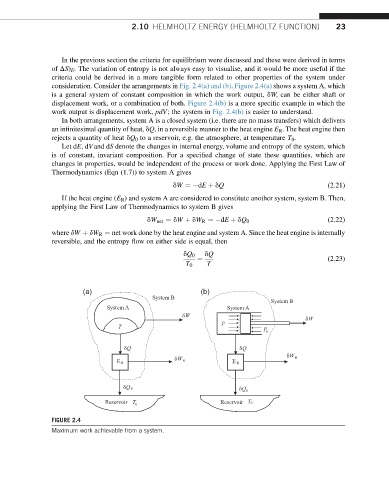
consideration. Consider the arrangements in Fig. 2.4(a) and (b). Figure 2.4(a) shows a system A, which
is a general system of constant composition in which the work output, dW, can be either shaft or
displacement work, or a combination of both. Figure 2.4(b) is a more specific example in which the
work output is displacement work, pdV; the system in Fig. 2.4(b) is easier to understand.
In both arrangements, system A is a closed system (i.e. there are no mass transfers) which delivers
an infinitesimal quantity of heat, dQ, in a reversible manner to the heat engine E R . The heat engine then
rejects a quantity of heat dQ 0 to a reservoir, e.g. the atmosphere, at temperature T 0 .
Let dE,dVand dS denote the changes in internal energy, volume and entropy of the system, which
is of constant, invariant composition. For a specified change of state these quantities, which are
changes in properties, would be independent of the process or work done. Applying the First Law of
Thermodynamics (Eqn (1.7)) to system A gives
dW ¼ dE þ dQ (2.21)
If the heat engine (E R ) and system A are considered to constitute another system, system B. Then,
applying the First Law of Thermodynamics to system B gives
dW net ¼ dW þ dW R ¼ dE þ dQ 0 (2.22)
where dW þ dW R ¼ net work done by the heat engine and system A. Since the heat engine is internally
reversible, and the entropy flow on either side is equal, then
dQ 0 dQ
¼ (2.23)
T 0 T
(a) (b)
System B
System B
System A System A
δW δW
p
T p
0
δQ δQ
δW R
δW R
E R E R
δQ 0
δQ 0
Reservoir T Reservoir
0 T 0
FIGURE 2.4
Maximum work achievable from a system.