Page 46 - Advanced thermodynamics for engineers
P. 46
2.15 PROBLEMS 29
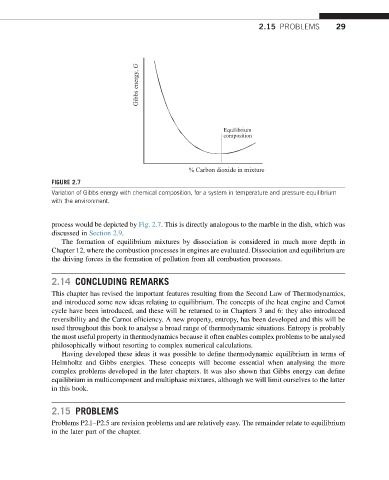
Gibbs energy, G
Equilibrium
composition
% Carbon dioxide in mixture
FIGURE 2.7
Variation of Gibbs energy with chemical composition, for a system in temperature and pressure equilibrium
with the environment.
process would be depicted by Fig. 2.7. This is directly analogous to the marble in the dish, which was
discussed in Section 2.9.
The formation of equilibrium mixtures by dissociation is considered in much more depth in
Chapter 12, where the combustion processes in engines are evaluated. Dissociation and equilibrium are
the driving forces in the formation of pollution from all combustion processes.
2.14 CONCLUDING REMARKS
This chapter has revised the important features resulting from the Second Law of Thermodynamics,
and introduced some new ideas relating to equilibrium. The concepts of the heat engine and Carnot
cycle have been introduced, and these will be returned to in Chapters 3 and 6: they also introduced
reversibility and the Carnot efficiency. A new property, entropy, has been developed and this will be
used throughout this book to analyse a broad range of thermodynamic situations. Entropy is probably
the most useful property in thermodynamics because it often enables complex problems to be analysed
philosophically without resorting to complex numerical calculations.
Having developed these ideas it was possible to define thermodynamic equilibrium in terms of
Helmholtz and Gibbs energies. These concepts will become essential when analysing the more
complex problems developed in the later chapters. It was also shown that Gibbs energy can define
equilibrium in multicomponent and multiphase mixtures, although we will limit ourselves to the latter
in this book.
2.15 PROBLEMS
Problems P2.1–P2.5 are revision problems and are relatively easy. The remainder relate to equilibrium
in the later part of the chapter.