Page 79 - Advanced thermodynamics for engineers
P. 79
64 CHAPTER 4 AVAILABILITY AND EXERGY
The change of specific (or molar) availability is
Da ¼ a 2 a 1 ¼ u 2 þ p 0 v 2 T 0 s 2 u 1 þ p 0 v 1 T 0 s 1
(4.12b)
¼ h 2 þ v 2 p 0 p 2 h 1 þ v 1 p 0 p 1 T 0 ðs 2 s 1 Þ
4.3 EXAMPLES
Example 4.3.1: reversible work from a piston-cylinder arrangement (this example is based on
Haywood (1980))
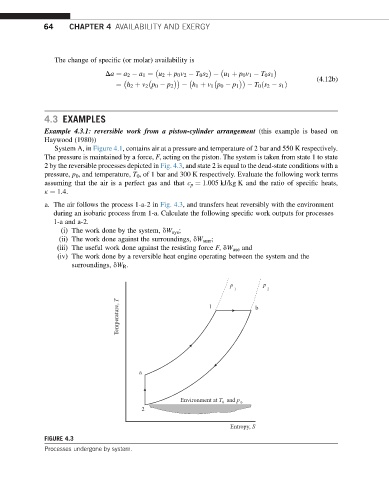
System A, in Figure 4.1, contains air at a pressure and temperature of 2 bar and 550 K respectively.
The pressure is maintained by a force, F, acting on the piston. The system is taken from state 1 to state
2 by the reversible processes depicted in Fig. 4.3, and state 2 is equal to the dead-state conditions with a
pressure, p 0 , and temperature, T 0 , of 1 bar and 300 K respectively. Evaluate the following work terms
assuming that the air is a perfect gas and that c p ¼ 1.005 kJ/kg K and the ratio of specific heats,
k ¼ 1.4.
a. The air follows the process 1-a-2 in Fig. 4.3, and transfers heat reversibly with the environment
during an isobaric process from 1-a. Calculate the following specific work outputs for processes
1-a and a-2.
(i) The work done by the system, dW sys ;
(ii) The work done against the surroundings, dW surr ;
(iii) The useful work done against the resisting force F, dW use and
(iv) The work done by a reversible heat engine operating between the system and the
surroundings, dW R .
p p
1 2
Temperature, T 1 b
a
Environment at T and p 0
0
2
Entropy, S
FIGURE 4.3
Processes undergone by system.