Page 88 - Advanced thermodynamics for engineers
P. 88
4.5 IRREVERSIBILITY 73
p = 7 bar
2
2
Temperature, T
2' p = 1 bar
1
T = 15 C 1
T = 5 C b a
0
m n
Entropy, S
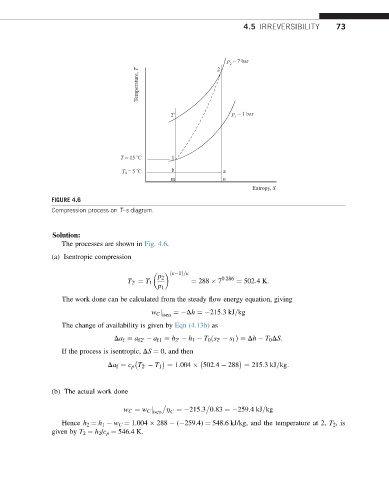
FIGURE 4.6
Compression process on T–s diagram.
Solution:
The processes are shown in Fig. 4.6.
(a) Isentropic compression
ðk 1Þ=k
p 2 0:286
T 2 ¼ T 1 ¼ 288 7 ¼ 502:4K:
0
p 1
The work done can be calculated from the steady flow energy equation, giving
w C j isen ¼ Dh ¼ 215:3kJ=kg
The change of availability is given by Eqn (4.13b) as
Da f ¼ a f2 a f1 ¼ h 2 h 1 T 0 ðs 2 s 1 Þ¼ Dh T 0 DS:
0
0
0
If the process is isentropic, DS ¼ 0, and then
Da f ¼ c p T 2 T 1 ¼ 1:004 502:4 288 ¼ 215:3kJ=kg:
0
(b) The actual work done
w C ¼ w C j isen h ¼ 215:3 0:83 ¼ 259:4kJ=kg
C
Hence h 2 ¼ h 1 w C ¼ 1.004 288 ( 259.4) ¼ 548.6 kJ/kg, and the temperature at 2, T 2 ,is
given by T 2 ¼ h 2 /c p ¼ 546.4 K.