Page 86 - Advanced thermodynamics for engineers
P. 86
4.5 IRREVERSIBILITY 71
change of availability and the work done, (c) the change of availability of the surroundings and (d) the
net loss of availability of the universe (i.e. the irreversibility).
Assume that the specific heat at constant pressure, c p ¼ 1.100 kJ/kg K, and that the ratio of specific
heats, k ¼ 1.35.
Solution:
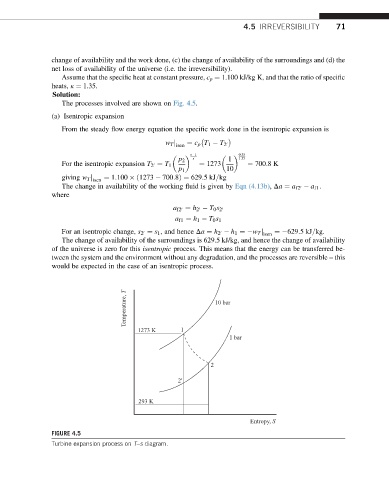
The processes involved are shown on Fig. 4.5.
(a) Isentropic expansion
From the steady flow energy equation the specific work done in the isentropic expansion is
w T j isen ¼ c p T 1 T 2 0
k 1 0:35
p 2 k 1 1:35
For the isentropic expansion T 2 ¼ T 1 ¼ 1273 ¼ 700:8K
0
p 1 10
giving w T j isen ¼ 1:100 ð1273 700:8Þ¼ 629:5kJ=kg
The change in availability of the working fluid is given by Eqn (4.13b), Da ¼ a f2 a f1 :
0
where
a f2 ¼ h 2 T 0 s 2 0
0
0
a f1 ¼ h 1 T 0 s 1
For an isentropic change, s 2 ¼ s 1 , and hence Da ¼ h 2 h 1 ¼ w T j isen ¼ 629:5kJ=kg.
0
0
The change of availability of the surroundings is 629.5 kJ/kg, and hence the change of availability
of the universe is zero for this isentropic process. This means that the energy can be transferred be-
tween the system and the environment without any degradation, and the processes are reversible – this
would be expected in the case of an isentropic process.
Temperature, T 10 bar
1273 K 1
1 bar
2
2'
293 K
Entropy, S
FIGURE 4.5
Turbine expansion process on T–s diagram.