Page 205 - Advances in bioenergy (2016)
P. 205
have elaborated about dry autothermal reforming of glycerol process, where they emphasize
for such operations based on four main thermodynamic parameters such as temperature,
pressure, OCGR [feed O /C (C of glycerol only) ratio], and CGR (feed CO to glycerol
2
2
70
ratio). The role of oxygen addition was to enhance the conversion of C –C products and to
1
4
promote the oxidation of coke and coke precursors from the catalyst surface by enhancing the
production of hydrogen, as supported by Swami and Abraham and Wang et al. 58,71-79 On the
basis of Damkohler number and Wagner criteria, Swami and Abraham concluded that the steam
reforming reaction for glycerol was surface-reaction controlled at lower temperatures (550–
650°C) and mass-transfer controlled at higher temperatures (700–850°C). 58,80
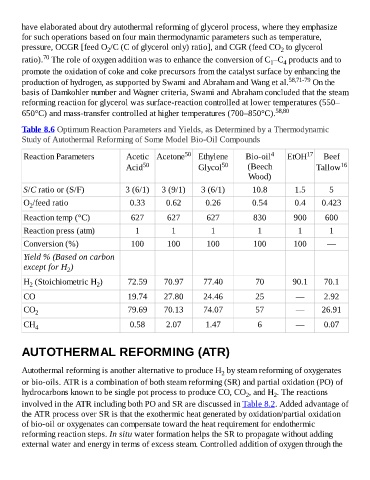
Table 8.6 Optimum Reaction Parameters and Yields, as Determined by a Thermodynamic
Study of Autothermal Reforming of Some Model Bio-Oil Compounds
Reaction Parameters Acetic Acetone 50 Ethylene Bio-oil 4 EtOH 17 Beef
Acid 50 Glycol 50 (Beech Tallow 16
Wood)
S/C ratio or (S/F) 3 (6/1) 3 (9/1) 3 (6/1) 10.8 1.5 5
O /feed ratio 0.33 0.62 0.26 0.54 0.4 0.423
2
Reaction temp (°C) 627 627 627 830 900 600
Reaction press (atm) 1 1 1 1 1 1
Conversion (%) 100 100 100 100 100 —
Yield % (Based on carbon
except for H )
2
H (Stoichiometric H ) 72.59 70.97 77.40 70 90.1 70.1
2 2
CO 19.74 27.80 24.46 25 — 2.92
CO 2 79.69 70.13 74.07 57 — 26.91
CH 4 0.58 2.07 1.47 6 — 0.07
AUTOTHERMAL REFORMING (ATR)
Autothermal reforming is another alternative to produce H by steam reforming of oxygenates
2
or bio-oils. ATR is a combination of both steam reforming (SR) and partial oxidation (PO) of
hydrocarbons known to be single pot process to produce CO, CO , and H . The reactions
2
2
involved in the ATR including both PO and SR are discussed in Table 8.2. Added advantage of
the ATR process over SR is that the exothermic heat generated by oxidation/partial oxidation
of bio-oil or oxygenates can compensate toward the heat requirement for endothermic
reforming reaction steps. In situ water formation helps the SR to propagate without adding
external water and energy in terms of excess steam. Controlled addition of oxygen through the