Page 203 - Advances in bioenergy (2016)
P. 203
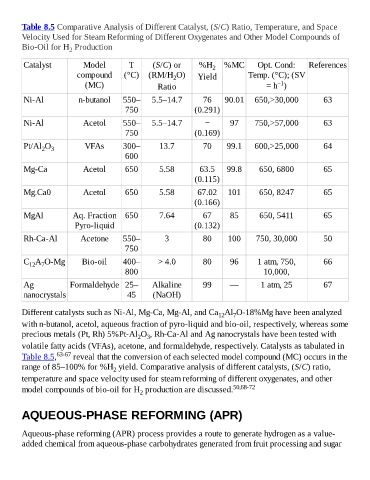
Table 8.5 Comparative Analysis of Different Catalyst, (S/C) Ratio, Temperature, and Space
Velocity Used for Steam Reforming of Different Oxygenates and Other Model Compounds of
Bio-Oil for H Production
2
Catalyst Model T (S/C) or %H 2 %MC Opt. Cond: References
compound (°C) (RM/H O) Yield Temp. (°C); (SV
2
−1
(MC) Ratio = h )
Ni-Al n-butanol 550– 5.5–14.7 76 90.01 650,>30,000 63
750 (0.291)
Ni-Al Acetol 550– 5.5–14.7 − 97 750,>57,000 63
750 (0.169)
Pt/Al O VFAs 300– 13.7 70 99.1 600,>25,000 64
2 3
600
Mg-Ca Acetol 650 5.58 63.5 99.8 650, 6800 65
(0.115)
Mg.Ca0 Acetol 650 5.58 67.02 101 650, 8247 65
(0.166)
MgAl Aq. Fraction 650 7.64 67 85 650, 5411 65
Pyro-liquid (0.132)
Rh-Ca-Al Acetone 550– 3 80 100 750, 30,000 50
750
C A O-Mg Bio-oil 400– > 4.0 80 96 1 atm, 750, 66
12 7
800 10,000,
Ag Formaldehyde 25– Alkaline 99 — 1 atm, 25 67
nanocrystals 45 (NaOH)
Different catalysts such as Ni-Al, Mg-Ca, Mg-Al, and Ca Al O-18%Mg have been analyzed
12
7
with n-butanol, acetol, aqueous fraction of pyro-liquid and bio-oil, respectively, whereas some
precious metals (Pt, Rh) 5%Pt-Al O , Rh-Ca-Al and Ag nanocrystals have been tested with
2 3
volatile fatty acids (VFAs), acetone, and formaldehyde, respectively. Catalysts as tabulated in
Table 8.5, 63-67 reveal that the conversion of each selected model compound (MC) occurs in the
range of 85–100% for %H yield. Comparative analysis of different catalysts, (S/C) ratio,
2
temperature and space velocity used for steam reforming of different oxygenates, and other
model compounds of bio-oil for H production are discussed. 50,68-72
2
AQUEOUS-PHASE REFORMING (APR)
Aqueous-phase reforming (APR) process provides a route to generate hydrogen as a value-
added chemical from aqueous-phase carbohydrates generated from fruit processing and sugar