Page 199 - Advances in bioenergy (2016)
P. 199
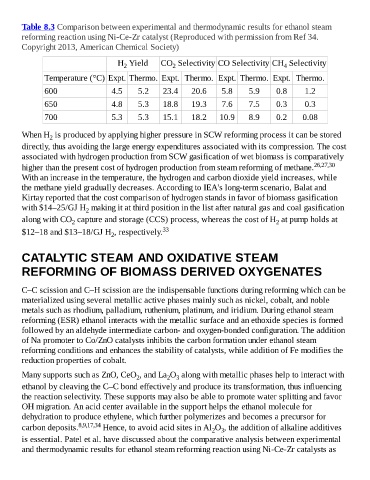
Table 8.3 Comparison between experimental and thermodynamic results for ethanol steam
reforming reaction using Ni-Ce-Zr catalyst (Reproduced with permission from Ref 34.
Copyright 2013, American Chemical Society)
H Yield CO Selectivity CO Selectivity CH Selectivity
4
2
2
Temperature (°C) Expt. Thermo. Expt. Thermo. Expt. Thermo. Expt. Thermo.
600 4.5 5.2 23.4 20.6 5.8 5.9 0.8 1.2
650 4.8 5.3 18.8 19.3 7.6 7.5 0.3 0.3
700 5.3 5.3 15.1 18.2 10.9 8.9 0.2 0.08
When H is produced by applying higher pressure in SCW reforming process it can be stored
2
directly, thus avoiding the large energy expenditures associated with its compression. The cost
associated with hydrogen production from SCW gasification of wet biomass is comparatively
higher than the present cost of hydrogen production from steam reforming of methane. 26,27,30
With an increase in the temperature, the hydrogen and carbon dioxide yield increases, while
the methane yield gradually decreases. According to IEA's long-term scenario, Balat and
Kirtay reported that the cost comparison of hydrogen stands in favor of biomass gasification
with $14–25/GJ H making it at third position in the list after natural gas and coal gasification
2
along with CO capture and storage (CCS) process, whereas the cost of H at pump holds at
2 2
$12–18 and $13–18/GJ H , respectively. 33
2
CATALYTIC STEAM AND OXIDATIVE STEAM
REFORMING OF BIOMASS DERIVED OXYGENATES
C–C scission and C–H scission are the indispensable functions during reforming which can be
materialized using several metallic active phases mainly such as nickel, cobalt, and noble
metals such as rhodium, palladium, ruthenium, platinum, and iridium. During ethanol steam
reforming (ESR) ethanol interacts with the metallic surface and an ethoxide species is formed
followed by an aldehyde intermediate carbon- and oxygen-bonded configuration. The addition
of Na promoter to Co/ZnO catalysts inhibits the carbon formation under ethanol steam
reforming conditions and enhances the stability of catalysts, while addition of Fe modifies the
reduction properties of cobalt.
Many supports such as ZnO, CeO , and La O along with metallic phases help to interact with
2
2 3
ethanol by cleaving the C–C bond effectively and produce its transformation, thus influencing
the reaction selectivity. These supports may also be able to promote water splitting and favor
OH migration. An acid center available in the support helps the ethanol molecule for
dehydration to produce ethylene, which further polymerizes and becomes a precursor for
carbon deposits. 8,9,17,34 Hence, to avoid acid sites in Al O , the addition of alkaline additives
2 3
is essential. Patel et al. have discussed about the comparative analysis between experimental
and thermodynamic results for ethanol steam reforming reaction using Ni-Ce-Zr catalysts as