Page 219 - Advances in bioenergy (2016)
P. 219
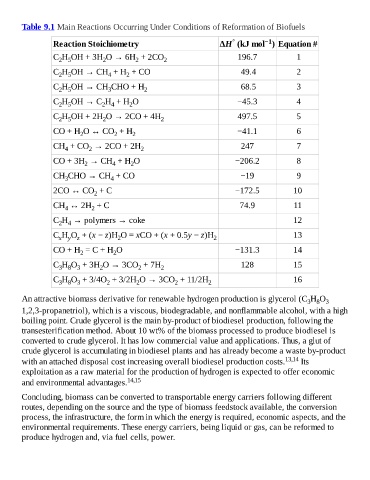
Table 9.1 Main Reactions Occurring Under Conditions of Reformation of Biofuels
°
−1
Reaction Stoichiometry ΔH (kJ mol ) Equation #
C H OH + 3H O → 6H + 2CO 2 196.7 1
2
2 5
2
C H OH → CH + H + CO 49.4 2
2
4
2 5
C H OH → CH CHO + H 2 68.5 3
2 5
3
C H OH → C H + H O −45.3 4
2 4
2
2 5
C H OH + 2H O → 2CO + 4H 2 497.5 5
2 5
2
CO + H O ↔ CO + H 2 −41.1 6
2
2
CH + CO → 2CO + 2H 2 247 7
2
4
CO + 3H → CH + H O −206.2 8
2
2
4
CH CHO → CH + CO −19 9
3 4
2CO ↔ CO + C −172.5 10
2
CH ↔ 2H + C 74.9 11
4 2
C H → polymers → coke 12
2 4
C H O + (x − z)H O = xCO + (x + 0.5y − z)H 13
x y z 2 2
CO + H = C + H O −131.3 14
2
2
C H O + 3H O → 3CO + 7H 2 128 15
2
2
3 8 3
C H O + 3/4O + 3/2H O → 3CO + 11/2H 2 16
2
2
2
3 8 3
An attractive biomass derivative for renewable hydrogen production is glycerol (C H O
3 8 3
1,2,3-propanetriol), which is a viscous, biodegradable, and nonflammable alcohol, with a high
boiling point. Crude glycerol is the main by-product of biodiesel production, following the
transesterification method. About 10 wt% of the biomass processed to produce biodiesel is
converted to crude glycerol. It has low commercial value and applications. Thus, a glut of
crude glycerol is accumulating in biodiesel plants and has already become a waste by-product
with an attached disposal cost increasing overall biodiesel production costs. 13,14 Its
exploitation as a raw material for the production of hydrogen is expected to offer economic
and environmental advantages. 14,15
Concluding, biomass can be converted to transportable energy carriers following different
routes, depending on the source and the type of biomass feedstock available, the conversion
process, the infrastructure, the form in which the energy is required, economic aspects, and the
environmental requirements. These energy carriers, being liquid or gas, can be reformed to
produce hydrogen and, via fuel cells, power.