Page 224 - Advances in bioenergy (2016)
P. 224
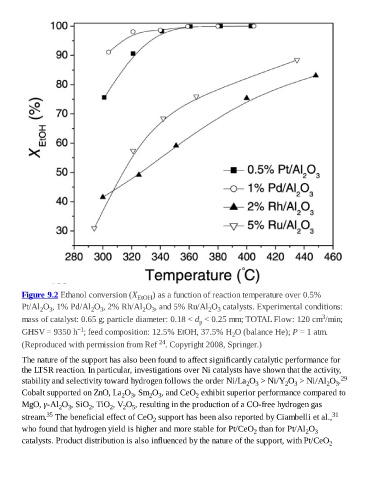
Figure 9.2 Ethanol conversion (X EtOH ) as a function of reaction temperature over 0.5%
Pt/Al O , 1% Pd/Al O , 2% Rh/Al O , and 5% Ru/Al O catalysts. Experimental conditions:
2 3 2 3 2 3 2 3
3
mass of catalyst: 0.65 g; particle diameter: 0.18 < d < 0.25 mm; TOTAL Flow: 120 cm /min;
p
−1
GHSV = 9350 h ; feed composition: 12.5% EtOH, 37.5% H O (balance He); P = 1 atm.
2
24
(Reproduced with permission from Ref . Copyright 2008, Springer.)
The nature of the support has also been found to affect significantly catalytic performance for
the LTSR reaction. In particular, investigations over Ni catalysts have shown that the activity,
stability and selectivity toward hydrogen follows the order Ni/La O > Ni/Y O > Ni/Al O . 29
2 3
2 3
2 3
Cobalt supported on ZnO, La O , Sm O , and CeO exhibit superior performance compared to
2 3
2
2 3
MgO, γ-Al O , SiO , TiO , V O , resulting in the production of a CO-free hydrogen gas
2
2 3
2 5
2
35
stream. The beneficial effect of CeO support has been also reported by Ciambelli et al., 31
2
who found that hydrogen yield is higher and more stable for Pt/CeO than for Pt/Al O
2
2 3
catalysts. Product distribution is also influenced by the nature of the support, with Pt/CeO 2