Page 60 - Advances in bioenergy (2016)
P. 60
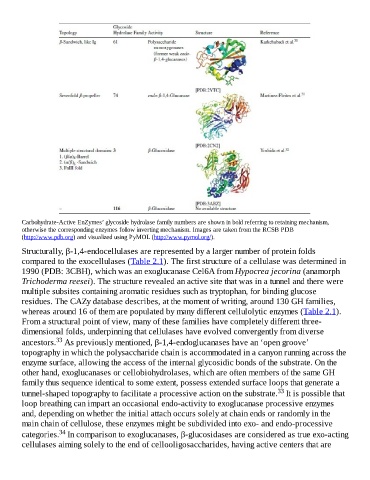
Carbohydrate-Active EnZymes’ glycoside hydrolase family numbers are shown in bold referring to retaining mechanism,
otherwise the corresponding enzymes follow inverting mechanism. Images are taken from the RCSB PDB
(http://www.pdb.org) and visualized using PyMOL (http://www.pymol.org/).
Structurally, β-1,4-endocellulases are represented by a larger number of protein folds
compared to the exocellulases (Table 2.1). The first structure of a cellulase was determined in
1990 (PDB: 3CBH), which was an exoglucanase Cel6A from Hypocrea jecorina (anamorph
Trichoderma reesei). The structure revealed an active site that was in a tunnel and there were
multiple subsites containing aromatic residues such as tryptophan, for binding glucose
residues. The CAZy database describes, at the moment of writing, around 130 GH families,
whereas around 16 of them are populated by many different cellulolytic enzymes (Table 2.1).
From a structural point of view, many of these families have completely different three-
dimensional folds, underpinning that cellulases have evolved convergently from diverse
33
ancestors. As previously mentioned, β-1,4-endoglucanases have an ‘open groove’
topography in which the polysaccharide chain is accommodated in a canyon running across the
enzyme surface, allowing the access of the internal glycosidic bonds of the substrate. On the
other hand, exoglucanases or cellobiohydrolases, which are often members of the same GH
family thus sequence identical to some extent, possess extended surface loops that generate a
33
tunnel-shaped topography to facilitate a processive action on the substrate. It is possible that
loop breathing can impart an occasional endo-activity to exoglucanase processive enzymes
and, depending on whether the initial attach occurs solely at chain ends or randomly in the
main chain of cellulose, these enzymes might be subdivided into exo- and endo-processive
34
categories. In comparison to exoglucanases, β-glucosidases are considered as true exo-acting
cellulases aiming solely to the end of cellooligosaccharides, having active centers that are