Page 168 - Advances in Forensic Applications of Mass Spectrometry - Jehuda Yinon
P. 168
1522_C04.fm Page 151 Thursday, November 13, 2003 9:54 AM
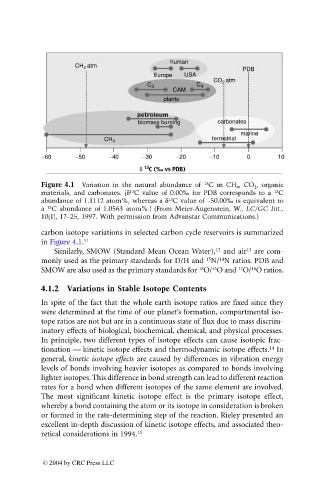
Figure 4.1 Variation in the natural abundance of C in CH 4 , CO 2 , organic
13
13
13
materials, and carbonates. (d C value of 0.00‰ for PDB corresponds to a C
13
abundance of 1.1112 atom%, whereas a d C value of –50.00‰ is equivalent to
a C abundance of 1.0563 atom%.) (From Meier-Augenstein, W., LC/GC Int.,
13
10(1), 17–25, 1997. With permission from Advanstar Communications.)
carbon isotope variations in selected carbon cycle reservoirs is summarized
in Figure 4.1. 11
13
12
Similarly, SMOW (Standard Mean Ocean Water), and air are com-
15
14
monly used as the primary standards for D/H and N/ N ratios. PDB and
17
16
16
18
SMOW are also used as the primary standards for O/ O and O/ O ratios.
4.1.2 Variations in Stable Isotope Contents
In spite of the fact that the whole earth isotope ratios are fixed since they
were determined at the time of our planet’s formation, compartmental iso-
tope ratios are not but are in a continuous state of flux due to mass discrim-
inatory effects of biological, biochemical, chemical, and physical processes.
In principle, two different types of isotope effects can cause isotopic frac-
14
tionation — kinetic isotope effects and thermodynamic isotope effects. In
general, kinetic isotope effects are caused by differences in vibration energy
levels of bonds involving heavier isotopes as compared to bonds involving
lighter isotopes. This difference in bond strength can lead to different reaction
rates for a bond when different isotopes of the same element are involved.
The most significant kinetic isotope effect is the primary isotope effect,
whereby a bond containing the atom or its isotope in consideration is broken
or formed in the rate-determining step of the reaction. Rieley presented an
excellent in-depth discussion of kinetic isotope effects, and associated theo-
retical considerations in 1994. 15
© 2004 by CRC Press LLC