Page 178 - Advances in Forensic Applications of Mass Spectrometry - Jehuda Yinon
P. 178
1522_C04.fm Page 161 Thursday, November 13, 2003 9:54 AM
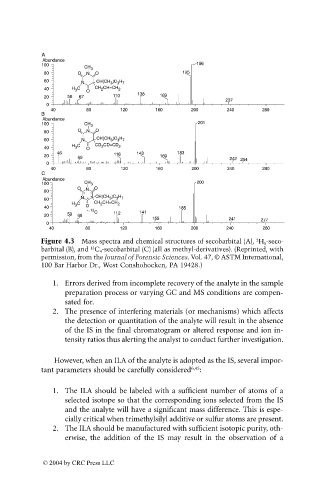
Figure 4.3 Mass spectra and chemical structures of secobarbital (A), H 5 -seco-
2
13
barbital (B), and C 4 -secobarbital (C) (all as methyl-derivatives). (Reprinted, with
permission, from the Journal of Forensic Sciences, Vol. 47, © ASTM International,
100 Bar Harbor Dr., West Conshohocken, PA 19428.)
1. Errors derived from incomplete recovery of the analyte in the sample
preparation process or varying GC and MS conditions are compen-
sated for.
2. The presence of interfering materials (or mechanisms) which affects
the detection or quantitation of the analyte will result in the absence
of the IS in the final chromatogram or altered response and ion in-
tensity ratios thus alerting the analyst to conduct further investigation.
However, when an ILA of the analyte is adopted as the IS, several impor-
tant parameters should be carefully considered 6,45 :
1. The ILA should be labeled with a sufficient number of atoms of a
selected isotope so that the corresponding ions selected from the IS
and the analyte will have a significant mass difference. This is espe-
cially critical when trimethylsilyl additive or sulfur atoms are present.
2. The ILA should be manufactured with sufficient isotopic purity, oth-
erwise, the addition of the IS may result in the observation of a
© 2004 by CRC Press LLC