Page 183 - Advances in Forensic Applications of Mass Spectrometry - Jehuda Yinon
P. 183
1522_C04.fm Page 166 Thursday, November 13, 2003 9:54 AM
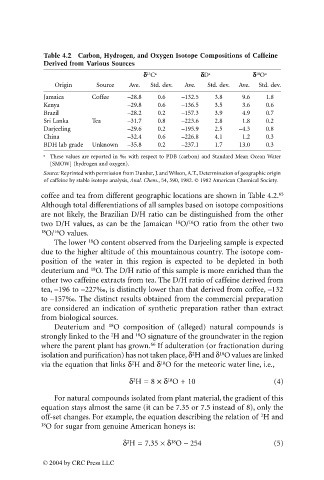
Table 4.2 Carbon, Hydrogen, and Oxygen Isotope Compositions of Caffeine
Derived from Various Sources
dd d d C a dd d dD a dd d d O a
13
18
Origin Source Ave. Std. dev. Ave. Std. dev. Ave. Std. dev.
Jamaica Coffee –28.8 0.6 –132.5 3.8 9.6 1.8
Kenya –29.8 0.6 –136.5 3.5 3.6 0.6
Brazil –28.2 0.2 –157.3 3.9 4.9 0.7
Sri Lanka Tea –31.7 0.8 –223.6 2.8 1.8 0.2
Darjeeling –29.6 0.2 –195.9 2.5 –4.3 0.8
China –32.4 0.6 –226.8 4.1 1.2 0.3
BDH lab grade Unknown –35.8 0.2 –237.1 1.7 13.0 0.3
a These values are reported in ‰ with respect to PDB (carbon) and Standard Mean Ocean Water
[SMOW] (hydrogen and oxygen).
Source: Reprinted with permission from Dunbar, J. and Wilson, A.T., Determination of geographic origin
of caffeine by stable isotope analysis, Anal. Chem., 54, 590, 1982. © 1982 American Chemical Society.
coffee and tea from different geographic locations are shown in Table 4.2. 65
Although total differentiations of all samples based on isotope compositions
are not likely, the Brazilian D/H ratio can be distinguished from the other
18
two D/H values, as can be the Jamaican O/ O ratio from the other two
16
16
18 O/ O values.
18
The lower O content observed from the Darjeeling sample is expected
due to the higher altitude of this mountainous country. The isotope com-
position of the water in this region is expected to be depleted in both
18
deuterium and O. The D/H ratio of this sample is more enriched than the
other two caffeine extracts from tea. The D/H ratio of caffeine derived from
tea, –196 to –227‰, is distinctly lower than that derived from coffee, –132
to –157‰. The distinct results obtained from the commercial preparation
are considered an indication of synthetic preparation rather than extract
from biological sources.
18
Deuterium and O composition of (alleged) natural compounds is
strongly linked to the H and O signature of the groundwater in the region
2
18
66
where the parent plant has grown. If adulteration (or fractionation during
isolation and purification) has not taken place, d H and d O values are linked
2
18
via the equation that links d H and d O for the meteoric water line, i.e.,
18
2
18
2
d H = 8 ¥ d O + 10 (4)
For natural compounds isolated from plant material, the gradient of this
equation stays almost the same (it can be 7.35 or 7.5 instead of 8), only the
2
off-set changes. For example, the equation describing the relation of H and
18 O for sugar from genuine American honeys is:
d H = 7.35 ¥ d O – 254 (5)
18
2
© 2004 by CRC Press LLC