Page 191 - Advances in Forensic Applications of Mass Spectrometry - Jehuda Yinon
P. 191
1522_C04.fm Page 174 Thursday, November 13, 2003 9:54 AM
13
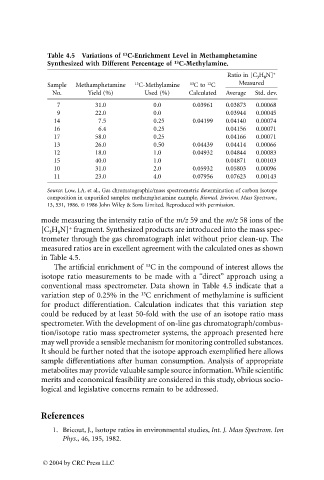
Table 4.5 Variations of C-Enrichment Level in Methamphetamine
Synthesized with Different Percentage of C-Methylamine.
13
Ratio in [C 3 H 8 N] +
Sample Methamphetamine 13 C-Methylamine 13 C to C Measured
12
No. Yield (%) Used (%) Calculated Average Std. dev.
7 31.0 0.0 0.03961 0.03873 0.00068
9 22.0 0.0 0.03944 0.00045
14 7.5 0.25 0.04199 0.04140 0.00074
16 6.4 0.25 0.04156 0.00071
17 58.0 0.25 0.04166 0.00071
13 26.0 0.50 0.04439 0.04414 0.00066
12 18.0 1.0 0.04932 0.04844 0.00083
15 40.0 1.0 0.04871 0.00103
10 31.0 2.0 0.05932 0.05803 0.00096
11 23.0 4.0 0.07956 0.07623 0.00143
Source: Low, I.A. et al., Gas chromatographic/mass spectrometric determination of carbon Isotope
composition in unpurified samples: methamphetamine example, Biomed. Environ. Mass Spectrom.,
13, 531, 1986. © 1986 John Wiley & Sons Limited. Reproduced with permission.
mode measuring the intensity ratio of the m/z 59 and the m/z 58 ions of the
+
[C H N] fragment. Synthesized products are introduced into the mass spec-
3 8
trometer through the gas chromatograph inlet without prior clean-up. The
measured ratios are in excellent agreement with the calculated ones as shown
in Table 4.5.
13
The artificial enrichment of C in the compound of interest allows the
isotope ratio measurements to be made with a “direct” approach using a
conventional mass spectrometer. Data shown in Table 4.5 indicate that a
variation step of 0.25% in the C enrichment of methylamine is sufficient
13
for product differentiation. Calculation indicates that this variation step
could be reduced by at least 50-fold with the use of an isotope ratio mass
spectrometer. With the development of on-line gas chromatograph/combus-
tion/isotope ratio mass spectrometer systems, the approach presented here
may well provide a sensible mechanism for monitoring controlled substances.
It should be further noted that the isotope approach exemplified here allows
sample differentiations after human consumption. Analysis of appropriate
metabolites may provide valuable sample source information. While scientific
merits and economical feasibility are considered in this study, obvious socio-
logical and legislative concerns remain to be addressed.
References
1. Bricout, J., Isotope ratios in environmental studies, Int. J. Mass Spectrom. Ion
Phys., 46, 195, 1982.
© 2004 by CRC Press LLC