Page 78 - Aerodynamics for Engineering Students
P. 78
Governing equations of fluid mechanics 61
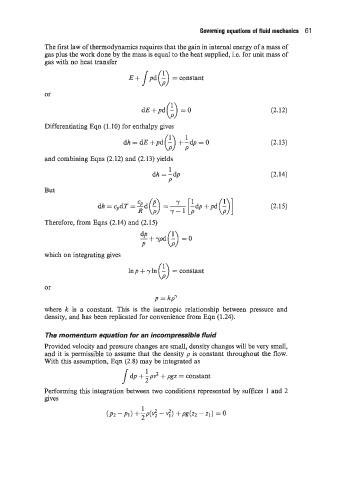
The first law of thermodynamics requires that the gain in internal energy of a mass of
gas plus the work done by the mass is equal to the heat supplied, i.e. for unit mass of
gas with no heat transfer
s (3
E+ pd - =constant
or
dE+pd(b) =o (2.12)
Differentiating Eqn (1 .lo) for enthalpy gives
(2.13)
and combining Eqns (2.12) and (2.13) yields
1
dh = -dp (2.14)
P
But
(2.15)
Therefore, from Eqns (2.14) and (2.15)
*+ypd(;) =o
P
which on integrating gives
1np + y In (b) = constant
or
p = kp^/
where k is a constant. This is the isentropic relationship between pressure and
density, and has been replicated for convenience from Eqn (1.24).
The momentum equation for an incompressible fluid
Provided velocity and pressure changes are small, density changes will be very small,
and it is permissible to assume that the density p is constant throughout the flow.
With this assumption, Eqn (2.8) may be integrated as
1
1 dp + zp? + pgz = constant
Performing this integration between two conditions represented by suffices 1 and 2
gives
1
(P2 -P1) +p(v; - vi) + PdZ2 -a) = 0