Page 157 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 157
5.1 Adsorption 131
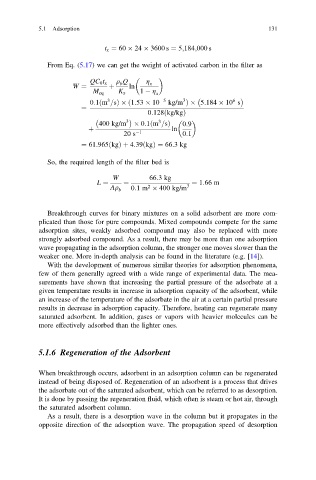
t x ¼ 60 24 3600 s ¼ 5;184;000 s
From Eq. (5.17) we can get the weight of activated carbon in the filter as
q Q g
QC 0 t x b x
W ¼ þ ln
M eq K x 1 g x
3
6
3
0:1m =sÞ ð1:53 10 5 kg/m Þ 5:184 10 s
ð
¼
ð
0:128 kg/kgÞ
3 3
400 kg/m 0:1m =sð Þ 0:9
þ ln
20 s 1 0:1
¼ 61:965 kgÞ þ 4:39 kgÞ ¼ 66:3kg
ð
ð
So, the required length of the filter bed is
W 66:3kg
L ¼ ¼ ¼ 1:66 m
Aq 0:1m 400 kg/m 3
2
b
Breakthrough curves for binary mixtures on a solid adsorbent are more com-
plicated than those for pure compounds. Mixed compounds compete for the same
adsorption sites, weakly adsorbed compound may also be replaced with more
strongly adsorbed compound. As a result, there may be more than one adsorption
wave propagating in the adsorption column, the stronger one moves slower than the
weaker one. More in-depth analysis can be found in the literature (e.g. [14]).
With the development of numerous similar theories for adsorption phenomena,
few of them generally agreed with a wide range of experimental data. The mea-
surements have shown that increasing the partial pressure of the adsorbate at a
given temperature results in increase in adsorption capacity of the adsorbent, while
an increase of the temperature of the adsorbate in the air at a certain partial pressure
results in decrease in adsorption capacity. Therefore, heating can regenerate many
saturated adsorbent. In addition, gases or vapors with heavier molecules can be
more effectively adsorbed than the lighter ones.
5.1.6 Regeneration of the Adsorbent
When breakthrough occurs, adsorbent in an adsorption column can be regenerated
instead of being disposed of. Regeneration of an adsorbent is a process that drives
the adsorbate out of the saturated adsorbent, which can be referred to as desorption.
It is done by passing the regeneration fluid, which often is steam or hot air, through
the saturated adsorbent column.
As a result, there is a desorption wave in the column but it propagates in the
opposite direction of the adsorption wave. The propagation speed of desorption