Page 169 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 169
5.2 Absorption 143
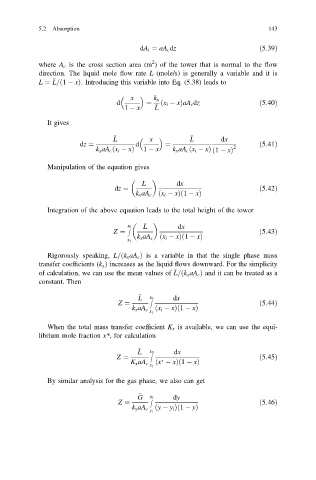
dA i ¼ aA c dz ð5:39Þ
2
where A c is the cross section area (m ) of the tower that is normal to the flow
direction. The liquid mole flow rate L (mole/s) is generally a variable and it is
L ¼ L= 1 xÞ: Introducing this variable into Eq. (5.38) leads to
ð
x k x
d ¼ ð x i xÞaA c dz ð5:40Þ
L
1 x
It gives
x dx
L
L
dz ¼ d ¼ 2 ð5:41Þ
k x aA c x i xð Þ 1 x k x aA c x i xð Þ 1 xð Þ
Manipulation of the equation gives
dx
L
dz ¼ ð5:42Þ
k x aA c ðx i xÞð1 xÞ
Integration of the above equation leads to the total height of the tower
x
Z 0 L dx
Z ¼ ð5:43Þ
k x aA c ð x i xÞ 1 xð Þ
x 1
Rigorously speaking, L= k x aA c Þ is a variable in that the single phase mass
ð
transfer coefficients (k x Þ increases as the liquid flows downward. For the simplicity
of calculation, we can use the mean values of L= k x aA c Þ and it can be treated as a
ð
constant. Then
x Z 0 dx
L
Z ¼ ð5:44Þ
ð x i xÞ 1 xð Þ
k x aA c x 1
When the total mass transfer coefficient K x is available, we can use the equi-
librium mole fraction x*, for calculation
x Z 0 dx
L
Z ¼ ð5:45Þ
ð x xÞ 1 xð Þ
K x aA c x 1
By similar analysis for the gas phase, we also can get
G y Z 0 dy
Z ¼ ð5:46Þ
ð y y i Þ 1 yð Þ
k y aA c y 1