Page 226 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 226
202 7 Combustion Process and Air Emission Formation
CaC 2 O 4 •H 2 O CaC 2 O 4 + H 2 O
CaC O CaCO + CO
2 4 3
CaCO CaO+ CO
3 2
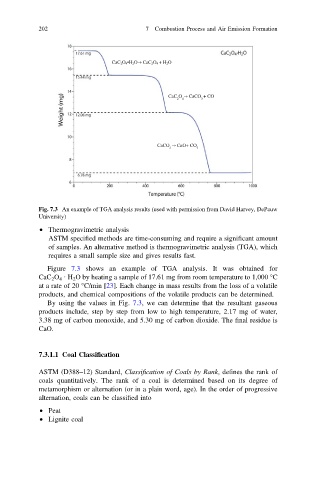
Fig. 7.3 An example of TGA analysis results (used with permission from David Harvey, DePauw
University)
• Thermogravimetric analysis
ASTM specified methods are time-consuming and require a significant amount
of samples. An alternative method is thermogravimetric analysis (TGA), which
requires a small sample size and gives results fast.
Figure 7.3 shows an example of TGA analysis. It was obtained for
CaC 2 O 4 · H 2 O by heating a sample of 17.61 mg from room temperature to 1,000 °C
at a rate of 20 °C/min [23]. Each change in mass results from the loss of a volatile
products, and chemical compositions of the volatile products can be determined.
By using the values in Fig. 7.3, we can determine that the resultant gaseous
products include, step by step from low to high temperature, 2.17 mg of water,
3.38 mg of carbon monoxide, and 5.30 mg of carbon dioxide. The final residue is
CaO.
7.3.1.1 Coal Classification
ASTM (D388–12) Standard, Classification of Coals by Rank,defines the rank of
coals quantitatively. The rank of a coal is determined based on its degree of
metamorphism or alternation (or in a plain word, age). In the order of progressive
alternation, coals can be classified into
• Peat
• Lignite coal