Page 225 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 225
7.3 Solid Fuel Combustion 201
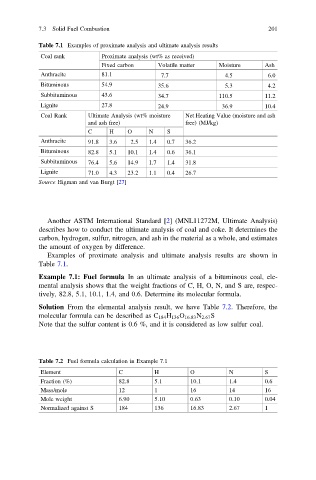
Table 7.1 Examples of proximate analysis and ultimate analysis results
Coal rank Proximate analysis (wt% as received)
Fixed carbon Volatile matter Moisture Ash
Anthracite 81.1 7.7 4.5 6.0
Bituminous 54.9 35.6 5.3 4.2
Subbituminous 43.6 34.7 110.5 11.2
Lignite 27.8 24.9 36.9 10.4
Coal Rank Ultimate Analysis (wt% moisture Net Heating Value (moisture and ash
and ash free) free) (MJ/kg)
C H O N S
Anthracite 91.8 3.6 2.5 1.4 0.7 36.2
Bituminous 82.8 5.1 10.1 1.4 0.6 36.1
Subbituminous 76.4 5.6 14.9 1.7 1.4 31.8
Lignite 71.0 4.3 23.2 1.1 0.4 26.7
Source Higman and van Burgt [27]
Another ASTM International Standard [2] (MNL11272M, Ultimate Analysis)
describes how to conduct the ultimate analysis of coal and coke. It determines the
carbon, hydrogen, sulfur, nitrogen, and ash in the material as a whole, and estimates
the amount of oxygen by difference.
Examples of proximate analysis and ultimate analysis results are shown in
Table 7.1.
Example 7.1: Fuel formula In an ultimate analysis of a bituminous coal, ele-
mental analysis shows that the weight fractions of C, H, O, N, and S are, respec-
tively, 82.8, 5.1, 10.1, 1.4, and 0.6. Determine its molecular formula.
Solution From the elemental analysis result, we have Table 7.2. Therefore, the
molecular formula can be described as C 184 H 136 O 16:83 N 2:67 S
Note that the sulfur content is 0.6 %, and it is considered as low sulfur coal.
Table 7.2 Fuel formula calculation in Example 7.1
Element C H O N S
Fraction (%) 82.8 5.1 10.1 1.4 0.6
Mass/mole 12 1 16 14 16
Mole weight 6.90 5.10 0.63 0.10 0.04
Normalized against S 184 136 16.83 2.67 1