Page 224 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 224
200 7 Combustion Process and Air Emission Formation
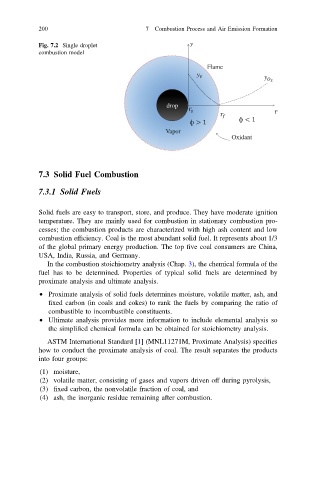
Fig. 7.2 Single droplet
combustion model
Flame
drop
Vapor
Oxidant
7.3 Solid Fuel Combustion
7.3.1 Solid Fuels
Solid fuels are easy to transport, store, and produce. They have moderate ignition
temperature. They are mainly used for combustion in stationary combustion pro-
cesses; the combustion products are characterized with high ash content and low
combustion efficiency. Coal is the most abundant solid fuel. It represents about 1/3
of the global primary energy production. The top five coal consumers are China,
USA, India, Russia, and Germany.
In the combustion stoichiometry analysis (Chap. 3), the chemical formula of the
fuel has to be determined. Properties of typical solid fuels are determined by
proximate analysis and ultimate analysis.
• Proximate analysis of solid fuels determines moisture, volatile matter, ash, and
fixed carbon (in coals and cokes) to rank the fuels by comparing the ratio of
combustible to incombustible constituents.
• Ultimate analysis provides more information to include elemental analysis so
the simplified chemical formula can be obtained for stoichiometry analysis.
ASTM International Standard [1] (MNL11271M, Proximate Analysis) specifies
how to conduct the proximate analysis of coal. The result separates the products
into four groups:
(1) moisture,
(2) volatile matter, consisting of gases and vapors driven off during pyrolysis,
(3) fixed carbon, the nonvolatile fraction of coal, and
(4) ash, the inorganic residue remaining after combustion.