Page 246 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 246
222 7 Combustion Process and Air Emission Formation
Ash
Char formation
Coal Particulate,
HgCl 2
HgO, HgSO 3,
HgS
Combustion
Vaporization
Gas-phase oxidation
T = 759-900 K
Adsorption
Hg HgCl 2
T>400K
Catalytic oxidation
T = 400-600 K
Vapor phase
mercury
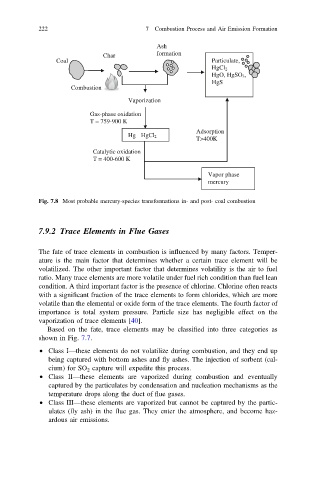
Fig. 7.8 Most probable mercury-species transformations in- and post- coal combustion
7.9.2 Trace Elements in Flue Gases
The fate of trace elements in combustion is influenced by many factors. Temper-
ature is the main factor that determines whether a certain trace element will be
volatilized. The other important factor that determines volatility is the air to fuel
ratio. Many trace elements are more volatile under fuel rich condition than fuel lean
condition. A third important factor is the presence of chlorine. Chlorine often reacts
with a significant fraction of the trace elements to form chlorides, which are more
volatile than the elemental or oxide form of the trace elements. The fourth factor of
importance is total system pressure. Particle size has negligible effect on the
vaporization of trace elements [40].
Based on the fate, trace elements may be classified into three categories as
shown in Fig. 7.7.
• Class I—these elements do not volatilize during combustion, and they end up
being captured with bottom ashes and fly ashes. The injection of sorbent (cal-
cium) for SO 2 capture will expedite this process.
• Class II—these elements are vaporized during combustion and eventually
captured by the particulates by condensation and nucleation mechanisms as the
temperature drops along the duct of flue gases.
• Class III—these elements are vaporized but cannot be captured by the partic-
ulates (fly ash) in the flue gas. They enter the atmosphere, and become haz-
ardous air emissions.