Page 271 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 271
8.4 Biofuels 247
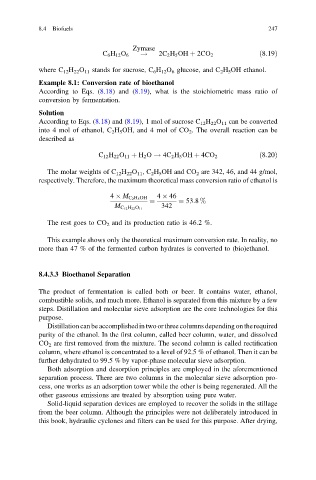
Zymase
C 6 H 12 O 6 ! 2C 2 H 5 OH þ 2CO 2 ð8:19Þ
where C 12 H 22 O 11 stands for sucrose, C 6 H 12 O 6 glucose, and C 2 H 5 OH ethanol.
Example 8.1: Conversion rate of bioethanol
According to Eqs. (8.18) and (8.19), what is the stoichiometric mass ratio of
conversion by fermentation.
Solution
According to Eqs. (8.18) and (8.19), 1 mol of sucrose C 12 H 22 O 11 can be converted
into 4 mol of ethanol, C 2 H 5 OH, and 4 mol of CO 2 . The overall reaction can be
described as
C 12 H 22 O 11 þ H 2 O ! 4C 2 H 5 OH þ 4CO 2 ð8:20Þ
The molar weights of C 12 H 22 O 11 ,C 2 H 5 OH and CO 2 are 342, 46, and 44 g/mol,
respectively. Therefore, the maximum theoretical mass conversion ratio of ethanol is
4 M C 2 H 5 OH 4 46
¼ ¼ 53:8 %
342
M C 12 H 22 O 11
The rest goes to CO 2 and its production ratio is 46.2 %.
This example shows only the theoretical maximum conversion rate. In reality, no
more than 47 % of the fermented carbon hydrates is converted to (bio)ethanol.
8.4.3.3 Bioethanol Separation
The product of fermentation is called both or beer. It contains water, ethanol,
combustible solids, and much more. Ethanol is separated from this mixture by a few
steps. Distillation and molecular sieve adsorption are the core technologies for this
purpose.
Distillation can beaccomplished in two or three columns dependingontherequired
purity of the ethanol. In the first column, called beer column, water, and dissolved
CO 2 are first removed from the mixture. The second column is called rectification
column, where ethanol is concentrated to a level of 92.5 % of ethanol. Then it can be
further dehydrated to 99.5 % by vapor-phase molecular sieve adsorption.
Both adsorption and desorption principles are employed in the aforementioned
separation process. There are two columns in the molecular sieve adsorption pro-
cess, one works as an adsorption tower while the other is being regenerated. All the
other gaseous emissions are treated by absorption using pure water.
Solid-liquid separation devices are employed to recover the solids in the stillage
from the beer column. Although the principles were not deliberately introduced in
this book, hydraulic cyclones and filters can be used for this purpose. After drying,