Page 273 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 273
8.4 Biofuels 249
The third parameter of concern is the dielectric constant of hot compressed
water. The dielectric constant of water decreases with the increase of temperature.
As a result, hot compressed water is much less polar than normal water. It performs
more like an organic solvent. The decreasing dielectric constant of hot compressed
water is likely to affect organic reactions. One benefit of the changing dielectric
constant of water is that it works like an organic solvent during reaction conditions,
and it returns to its normal polarity after cooling. This unique property is believed to
be beneficial to the production and voluntary separation of alkanes from biomass
[11].
The changing reaction environment around critical point approach causes a
significant change in reaction mechanisms of hydrothermal conversion of biomass
[7, 28]. Both ionic and free radical reactions may take place in hydrothermal
conversion of biomass, the latter being preferred above the critical point.
Depending the status of hot compressed water it can convert biomass into both
gaseous and liquid fuels. Without catalyst, the higher the water temperature, the
more gas, and the less liquid fuels. As a result, hydrothermal conversion processes
are divided into hydrothermal liquefaction and hydrothermal gasification.
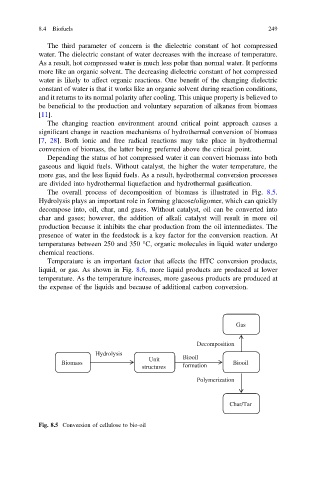
The overall process of decomposition of biomass is illustrated in Fig. 8.5.
Hydrolysis plays an important role in forming glucose/oligomer, which can quickly
decompose into, oil, char, and gases. Without catalyst, oil can be converted into
char and gases; however, the addition of alkali catalyst will result in more oil
production because it inhibits the char production from the oil intermediates. The
presence of water in the feedstock is a key factor for the conversion reaction. At
temperatures between 250 and 350 °C, organic molecules in liquid water undergo
chemical reactions.
Temperature is an important factor that affects the HTC conversion products,
liquid, or gas. As shown in Fig. 8.6, more liquid products are produced at lower
temperature. As the temperature increases, more gaseous products are produced at
the expense of the liquids and because of additional carbon conversion.
Gas
Decomposition
Hydrolysis
Unit Biooil
Biomass Biooil
structures formation
Polymerization
Char/Tar
Fig. 8.5 Conversion of cellulose to bio-oil