Page 274 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 274
250 8 Pre-combustion Air Emission Control
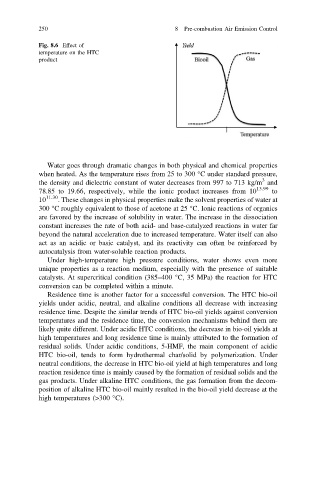
Fig. 8.6 Effect of
temperature on the HTC
product
Water goes through dramatic changes in both physical and chemical properties
when heated. As the temperature rises from 25 to 300 °C under standard pressure,
3
the density and dielectric constant of water decreases from 997 to 713 kg/m and
13.99
78.85 to 19.66, respectively, while the ionic product increases from 10 to
11.30
10 . These changes in physical properties make the solvent properties of water at
300 °C roughly equivalent to those of acetone at 25 °C. Ionic reactions of organics
are favored by the increase of solubility in water. The increase in the dissociation
constant increases the rate of both acid- and base-catalyzed reactions in water far
beyond the natural acceleration due to increased temperature. Water itself can also
act as an acidic or basic catalyst, and its reactivity can often be reinforced by
autocatalysis from water-soluble reaction products.
Under high-temperature high pressure conditions, water shows even more
unique properties as a reaction medium, especially with the presence of suitable
catalysts. At supercritical condition (385–400 °C, 35 MPa) the reaction for HTC
conversion can be completed within a minute.
Residence time is another factor for a successful conversion. The HTC bio-oil
yields under acidic, neutral, and alkaline conditions all decrease with increasing
residence time. Despite the similar trends of HTC bio-oil yields against conversion
temperatures and the residence time, the conversion mechanisms behind them are
likely quite different. Under acidic HTC conditions, the decrease in bio-oil yields at
high temperatures and long residence time is mainly attributed to the formation of
residual solids. Under acidic conditions, 5-HMF, the main component of acidic
HTC bio-oil, tends to form hydrothermal char/solid by polymerization. Under
neutral conditions, the decrease in HTC bio-oil yield at high temperatures and long
reaction residence time is mainly caused by the formation of residual solids and the
gas products. Under alkaline HTC conditions, the gas formation from the decom-
position of alkaline HTC bio-oil mainly resulted in the bio-oil yield decrease at the
high temperatures (>300 °C).