Page 327 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 327
304 10 Post-combustion Air Emission Control
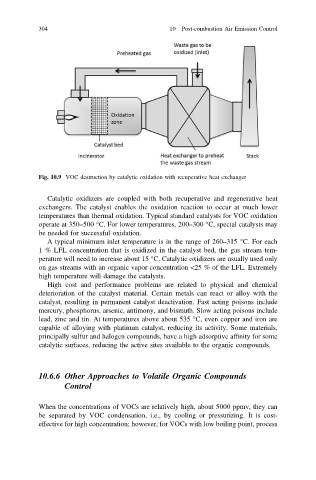
Fig. 10.9 VOC destruction by catalytic oxidation with recuperative heat exchanger
Catalytic oxidizers are coupled with both recuperative and regenerative heat
exchangers. The catalyst enables the oxidation reaction to occur at much lower
temperatures than thermal oxidation. Typical standard catalysts for VOC oxidation
operate at 350–500 °C. For lower temperatures, 200–300 °C, special catalysts may
be needed for successful oxidation.
A typical minimum inlet temperature is in the range of 260–315 °C. For each
1 % LFL concentration that is oxidized in the catalyst bed, the gas stream tem-
perature will need to increase about 15 °C. Catalytic oxidizers are usually used only
on gas streams with an organic vapor concentration <25 % of the LFL. Extremely
high temperature will damage the catalysts.
High cost and performance problems are related to physical and chemical
deterioration of the catalyst material. Certain metals can react or alloy with the
catalyst, resulting in permanent catalyst deactivation. Fast acting poisons include
mercury, phosphorus, arsenic, antimony, and bismuth. Slow acting poisons include
lead, zinc and tin. At temperatures above about 535 °C, even copper and iron are
capable of alloying with platinum catalyst, reducing its activity. Some materials,
principally sulfur and halogen compounds, have a high adsorptive affinity for some
catalytic surfaces, reducing the active sites available to the organic compounds.
10.6.6 Other Approaches to Volatile Organic Compounds
Control
When the concentrations of VOCs are relatively high, about 5000 ppmv, they can
be separated by VOC condensation, i.e., by cooling or pressurizing. It is cost-
effective for high concentration; however, for VOCs with low boiling point, process