Page 371 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 371
350 12 Carbon Capture and Storage
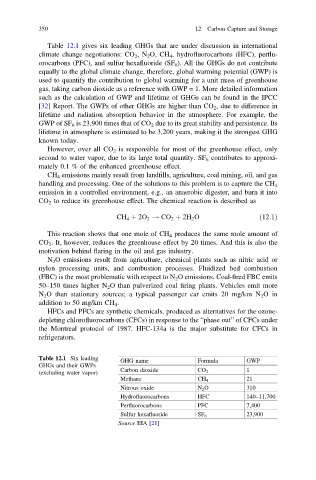
Table 12.1 gives six leading GHGs that are under discussion in international
climate change negotiations: CO 2 ,N 2 O, CH 4 , hydrofluorocarbons (HFC), perflu-
orocarbons (PFC), and sulfur hexafluoride (SF 6 ). All the GHGs do not contribute
equally to the global climate change, therefore, global warming potential (GWP) is
used to quantify the contribution to global warming for a unit mass of greenhouse
gas, taking carbon dioxide as a reference with GWP = 1. More detailed information
such as the calculation of GWP and lifetime of GHGs can be found in the IPCC
[32] Report. The GWPs of other GHGs are higher than CO 2 , due to difference in
lifetime and radiation absorption behavior in the atmosphere. For example, the
GWP of SF 6 is 23,900 times that of CO 2 due to its great stability and persistence. Its
lifetime in atmosphere is estimated to be 3,200 years, making it the strongest GHG
known today.
However, over all CO 2 is responsible for most of the greenhouse effect, only
second to water vapor, due to its large total quantity. SF 6 contributes to approxi-
mately 0.1 % of the enhanced greenhouse effect.
CH 4 emissions mainly result from landfills, agriculture, coal mining, oil, and gas
handling and processing. One of the solutions to this problem is to capture the CH 4
emission in a controlled environment, e.g., an anaerobic digester, and burn it into
CO 2 to reduce its greenhouse effect. The chemical reaction is described as
CH 4 þ 2O 2 ! CO 2 þ 2H 2 O ð12:1Þ
This reaction shows that one mole of CH 4 produces the same mole amount of
CO 2 . It, however, reduces the greenhouse effect by 20 times. And this is also the
motivation behind flaring in the oil and gas industry.
N 2 O emissions result from agriculture, chemical plants such as nitric acid or
nylon processing units, and combustion processes. Fluidized bed combustion
(FBC) is the most problematic with respect to N 2 O emissions. Coal-fired FBC emits
50–150 times higher N 2 O than pulverized coal firing plants. Vehicles emit more
N 2 O than stationary sources; a typical passenger car emits 20 mg/km N 2 Oin
addition to 50 mg/km CH 4 .
HFCs and PFCs are synthetic chemicals, produced as alternatives for the ozone-
depleting chlorofluorocarbons (CFCs) in response to the “phase out” of CFCs under
the Montreal protocol of 1987. HFC-134a is the major substitute for CFCs in
refrigerators.
Table 12.1 Six leading GHG name Formula GWP
GHGs and their GWPs
(excluding water vapor) Carbon dioxide CO 2 1
Methane CH 4 21
Nitrous oxide N 2 O 310
Hydrofluorocarbons HFC 140–11,700
Perfluorocarbons PFC 7,400
Sulfur hexafluoride SF 6 23,900
Source EIA [21]