Page 404 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 404
12.8 CO 2 Storage 383
12.8.2 Coal Bed Methane Recovery
Coal bed methane is the methane adsorbed in underground coals. Coal bed methane
recovery is then a methane desorption process based on pressure swing desorption.
The process is similar to EOR above; liquefied CO 2 is injected into a deep well,
which is drilled into the coal bed, to reduce the hydrostatic pressure. As a result,
methane is released from coal bed and transported to gas pipeline.
CO 2 injection also results in enhanced methane recovery from coal bed methane
by the competitive adsorption between CO 2 and methane. According to physical
adsorption mechanisms, CO 2 and methane compete for the adsorption sites in the
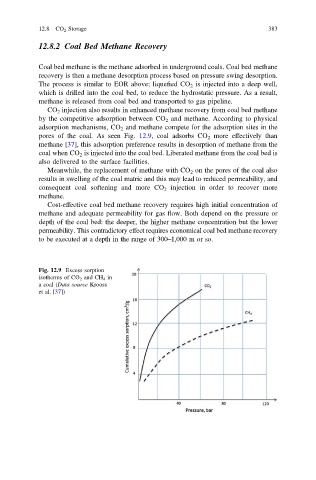
pores of the coal. As seen Fig. 12.9, coal adsorbs CO 2 more effectively than
methane [37], this adsorption preference results in desorption of methane from the
coal when CO 2 is injected into the coal bed. Liberated methane from the coal bed is
also delivered to the surface facilities.
Meanwhile, the replacement of methane with CO 2 on the pores of the coal also
results in swelling of the coal matric and this may lead to reduced permeability, and
consequent coal softening and more CO 2 injection in order to recover more
methane.
Cost-effective coal bed methane recovery requires high initial concentration of
methane and adequate permeability for gas flow. Both depend on the pressure or
depth of the coal bed: the deeper, the higher methane concentration but the lower
permeability. This contradictory effect requires economical coal bed methane recovery
to be executed at a depth in the range of 300–1,000 m or so.
Fig. 12.9 Excess sorption
isotherms of CO 2 and CH 4 in
a coal (Data source Krooss
et al. [37])