Page 399 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 399
378 12 Carbon Capture and Storage
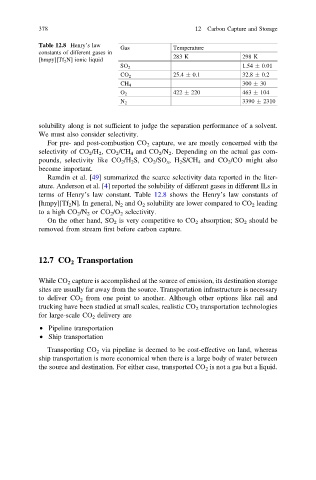
Table 12.8 Henry’s law
Gas Temperature
constants of different gases in
283 K 298 K
[hmpy][Tf 2 N] ionic liquid
1.54 0.01
SO 2
25.4 0.1 32.8 0.2
CO 2
CH 4 300 30
O 2 422 220 463 104
3390 2310
N 2
solubility along is not sufficient to judge the separation performance of a solvent.
We must also consider selectivity.
For pre- and post-combustion CO 2 capture, we are mostly concerned with the
selectivity of CO 2 /H 2 ,CO 2 /CH 4 and CO 2 /N 2 . Depending on the actual gas com-
pounds, selectivity like CO 2 /H 2 S, CO 2 /SO x ,H 2 S/CH 4 and CO 2 /CO might also
become important.
Ramdin et al. [49] summarized the scarce selectivity data reported in the liter-
ature. Anderson et al. [4] reported the solubility of different gases in different ILs in
terms of Henry’s law constant. Table 12.8 shows the Henry’s law constants of
[hmpy][Tf 2 N]. In general, N 2 and O 2 solubility are lower compared to CO 2 leading
to a high CO 2 /N 2 or CO 2 /O 2 selectivity.
On the other hand, SO 2 is very competitive to CO 2 absorption; SO 2 should be
removed from stream first before carbon capture.
12.7 CO 2 Transportation
While CO 2 capture is accomplished at the source of emission, its destination storage
sites are usually far away from the source. Transportation infrastructure is necessary
to deliver CO 2 from one point to another. Although other options like rail and
trucking have been studied at small scales, realistic CO 2 transportation technologies
for large-scale CO 2 delivery are
• Pipeline transportation
• Ship transportation
Transporting CO 2 via pipeline is deemed to be cost-effective on land, whereas
ship transportation is more economical when there is a large body of water between
the source and destination. For either case, transported CO 2 is not a gas but a liquid.