Page 484 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 484
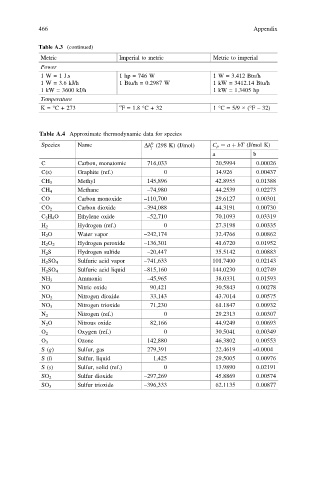
466 Appendix
Table A.3 (continued)
Metric Imperial to metric Metric to imperial
Power
1 W = 1 J.s 1 hp = 746 W 1 W = 3.412 Btu/h
1 W = 3.6 kJ/h 1 Btu/h = 0.2987 W 1 kW = 3412.14 Btu/h
1 kW = 3600 kJ/h 1 kW = 1.3405 hp
Temperature
K= °C + 273 o F = 1.8 °C + 32 1 °C = 5/9 × (°F – 32)
Table A.4 Approximate thermodynamic data for species
0
Species Name Dh (298 K) (J/mol) C p ¼ a þ bT (J/mol K)
f
a b
C Carbon, monatomic 716,033 20.5994 0.00026
C(s) Graphite (ref.) 0 14.926 0.00437
CH 3 Methyl 145,896 42.8955 0.01388
Methane –74,980 44.2539 0.02273
CH 4
CO Carbon monoxide –110,700 29.6127 0.00301
CO 2 Carbon dioxide –394,088 44.3191 0.00730
C 2 H 4 O Ethylene oxide –52,710 70.1093 0.03319
Hydrogen (ref.) 0 27.3198 0.00335
H 2
H 2 O Water vapor –242,174 32.4766 0.00862
H 2 O 2 Hydrogen peroxide –136,301 41.6720 0.01952
H 2 S Hydrogen sulfide –20,447 35.5142 0.00883
H 2 SO 4 Sulfuric acid vapor –741,633 101.7400 0.02143
H 2 SO 4 Sulfuric acid liquid –815,160 144.0230 0.02749
Ammonia –45,965 38.0331 0.01593
NH 3
NO Nitric oxide 90,421 30.5843 0.00278
NO 2 Nitrogen dioxide 33,143 43.7014 0.00575
Nitrogen trioxide 71,230 61.1847 0.00932
NO 3
Nitrogen (ref.) 0 29.2313 0.00307
N 2
N 2 O Nitrous oxide 82,166 44.9249 0.00693
Oxygen (ref.) 0 30.5041 0.00349
O 2
Ozone 142,880 46.3802 0.00553
O 3
S(g) Sulfur, gas 279,391 22.4619 –0.0004
S(l) Sulfur, liquid 1,425 29.5005 0.00976
S(s) Sulfur, solid (ref.) 0 13.9890 0.02191
Sulfur dioxide –297,269 45.8869 0.00574
SO 2
SO 3 Sulfur trioxide –396,333 62.1135 0.00877