Page 60 - Alternative Energy Systems in Building Design
P. 60
DYE-SENSITIZED SOLAR CELLS 37
Since the dye molecules are quite small in size, in order to capture a reasonable
amount of incoming solar rays or sunlight effectively, the layer of dye molecules is made
much thicker than the molecules themselves. To resolve this problem, a nanomaterial in
the form of a scaffold is used to hold or bundle large numbers of dye molecules in a
three-dimensional (3D) matrix. Bundles of large numbers of molecules thus provide a
large cell surface area. At present, this scaffolding is fabricated from semiconductor
material, which effectively serves double duty.
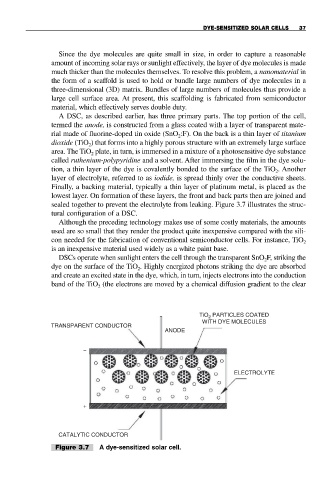
A DSC, as described earlier, has three primary parts. The top portion of the cell,
termed the anode, is constructed from a glass coated with a layer of transparent mate-
rial made of fluorine-doped tin oxide (SnO :F). On the back is a thin layer of titanium
2
dioxide (TiO ) that forms into a highly porous structure with an extremely large surface
2
area. The TiO plate, in turn, is immersed in a mixture of a photosensitive dye substance
2
called ruthenium-polypyridine and a solvent. After immersing the film in the dye solu-
tion, a thin layer of the dye is covalently bonded to the surface of the TiO . Another
2
layer of electrolyte, referred to as iodide, is spread thinly over the conductive sheets.
Finally, a backing material, typically a thin layer of platinum metal, is placed as the
lowest layer. On formation of these layers, the front and back parts then are joined and
sealed together to prevent the electrolyte from leaking. Figure 3.7 illustrates the struc-
tural configuration of a DSC.
Although the preceding technology makes use of some costly materials, the amounts
used are so small that they render the product quite inexpensive compared with the sili-
con needed for the fabrication of conventional semiconductor cells. For instance, TiO 2
is an inexpensive material used widely as a white paint base.
DSCs operate when sunlight enters the cell through the transparent SnO F, striking the
2
dye on the surface of the TiO . Highly energized photons striking the dye are absorbed
2
and create an excited state in the dye, which, in turn, injects electrons into the conduction
band of the TiO (the electrons are moved by a chemical diffusion gradient to the clear
2
TiO PARTICLES COATED
2
WITH DYE MOLECULES
TRANSPARENT CONDUCTOR
ANODE
−
ELECTROLYTE
+
CATALYTIC CONDUCTOR
Figure 3.7 A dye-sensitized solar cell.