Page 133 - An Introduction to Analytical Atomic Spectrometry - L. Ebdon
P. 133
Page 117
respectively, m is the electron mass, k is the Boltzmann constant, T is the temperature; h is Planck's
constant and E is the first ionization energy. In this case, ionization is effected by ion-atom and atom-
i
atom collisions, where the energy required for ionization is derived from thermal agitation of the
particles. The degree of ionization is dependent on the electron number density, the temperature and
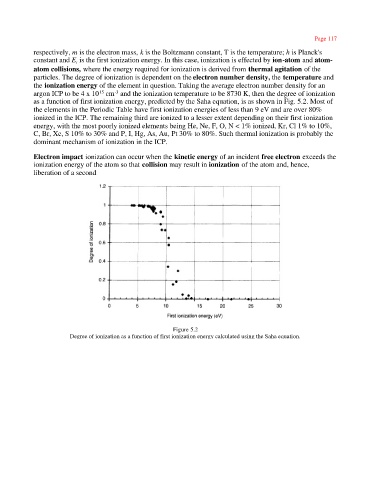
the ionization energy of the element in question. Taking the average electron number density for an
argon ICP to be 4 x 10 cm and the ionization temperature to be 8730 K, then the degree of ionization
-3
15
as a function of first ionization energy, predicted by the Saha equation, is as shown in Fig. 5.2. Most of
the elements in the Periodic Table have first ionization energies of less than 9 eV and are over 80%
ionized in the ICP. The remaining third are ionized to a lesser extent depending on their first ionization
energy, with the most poorly ionized elements being He, Ne, F, O, N < 1% ionized, Kr, Cl 1% to 10%,
C, Br, Xe, S 10% to 30% and P, I, Hg, As, Au, Pt 30% to 80%. Such thermal ionization is probably the
dominant mechanism of ionization in the ICP.
Electron impact ionization can occur when the kinetic energy of an incident free electron exceeds the
ionization energy of the atom so that collision may result in ionization of the atom and, hence,
liberation of a second
Figure 5.2
Degree of ionization as a function of first ionization energy calculated using the Saha equation.