Page 156 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 156
5-1 PRINCIPLES OF POTENTIOMETRIC MEASUREMENTS 141
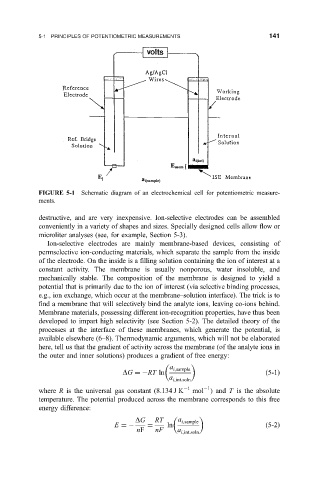
FIGURE 5-1 Schematic diagram of an electrochemical cell for potentiometric measure-
ments.
destructive, and are very inexpensive. Ion-selective electrodes can be assembled
conveniently in a variety of shapes and sizes. Specially designed cells allow ¯ow or
microliter analyses (see, for example, Section 5-3).
Ion-selective electrodes are mainly membrane-based devices, consisting of
permselective ion-conducting materials, which separate the sample from the inside
of the electrode. On the inside is a ®lling solution containing the ion of interest at a
constant activity. The membrane is usually nonporous, water insoluble, and
mechanically stable. The composition of the membrane is designed to yield a
potential that is primarily due to the ion of interest (via selective binding processes,
e.g., ion exchange, which occur at the membrane±solution interface). The trick is to
®nd a membrane that will selectively bind the analyte ions, leaving co-ions behind.
Membrane materials, possessing different ion-recognition properties, have thus been
developed to impart high selectivity (see Section 5-2). The detailed theory of the
processes at the interface of these membranes, which generate the potential, is
available elsewhere (6±8). Thermodynamic arguments, which will not be elaborated
here, tell us that the gradient of activity across the membrane (of the analyte ions in
the outer and inner solutions) produces a gradient of free energy:
a i;sample
DG RT ln
5-1
a i;int:soln
1
where R is the universal gas constant (8.134 J K 1 mol ) and T is the absolute
temperature. The potential produced across the membrane corresponds to this free
energy difference:
DG RT a i;sample
E ln
5-2
nF nF a i;int:soln