Page 161 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 161
146 POTENTIOMETRY
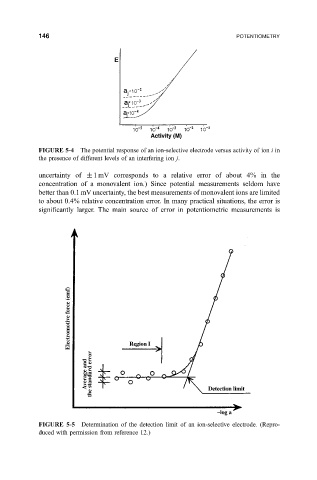
FIGURE 5-4 The potential response of an ion-selective electrode versus activity of ion i in
the presence of different levels of an interfering ion j.
uncertainty of 1 mV corresponds to a relative error of about 4% in the
concentration of a monovalent ion.) Since potential measurements seldom have
better than 0.1 mV uncertainty, the best measurements of monovalent ions are limited
to about 0.4% relative concentration error. In many practical situations, the error is
signi®cantly larger. The main source of error in potentiometric measurements is
FIGURE 5-5 Determination of the detection limit of an ion-selective electrode. (Repro-
duced with permission from reference 12.)