Page 175 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 175
160 POTENTIOMETRY
(Safety considerations dictate that cyanide measurements should be carried out in
strongly basic media.) The interference mechanism with silver-based solid-state ISEs
differs from that of ISEs described earlier. Depending on the K SP value, an excess of
the interfering ion may result in its deposition as silver salt on the membrane surface.
Removal of the interfering ®lm (by scrubbing) is thus required to restore the
electrode activity. Table 5-1 lists some solid-state electrodes from a commercial
source, along with their dynamic range and major interferences.
5-2.4 Coated-Wire Electrodes
Coated-wire electrodes (CWEs), introduced by Freiser in the mid-1970s, are
prepared by coating an appropriate polymeric ®lm directly onto a conductor
(Figure 5-16). The ion-responsive membrane is commonly based on poly(vinyl
chloride), while the conductor can be metallic (Pt, Ag, Cu) or graphite-based of any
conventional shape such as wire or disk. The conductor is usually dipped in a
solution of PVC and the active substance, and the resulting ®lm is allowed to air dry.
Other polymers and modi®ed polymers, including poly(acrylic acid) and modi®ed
poly(vinylbenzyl chloride) can also be useful for various applications. In addition to
the miniaturization capability, CWEs are extremely simple, inexpensive, and easy to
prepare and function well over the 10 5 ±10 1 M concentration range. The exact
mechanism of the behavior of the CWE continues to be a mystery, in view of the lack
of internal reference components. Coated-wire electrodes may suffer from reprodu-
cibility and long-term stability (drifting potential) problems. Nevertheless, such
devices have been found useful for various important applications, provided the
electrodes are calibrated periodically. Determinations of basic drugs (e.g., cocaine,
methodone) (42), amino acids (43), potassium, and sodium (44) represent some of
the useful applications of CWE. New concepts for preparing CWEs appear to
improve their analytical performance, particularly with respect to stability and
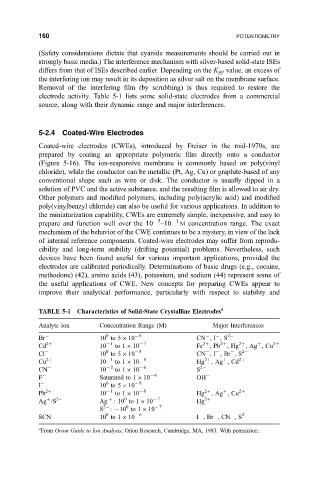
TABLE 5-1 Characteristics ofSolid-State Crystalline Electrodes a
Analyte Ion Concentration Range (M) Major Interferences
0
Br 10 to 5 10 6 CN ,I ,S 2
Cd 2 10 1 to 1 10 7 Fe 2 ,Pb 2 ,Hg 2 ,Ag ,Cu 2
0
Cl 10 to 5 10 5 CN ,I ,Br ,S 2
Cu 2 10 1 to 1 10 8 Hg 2 ,Ag ,Cd 2
CN 10 2 to 1 10 6 S 2
F Saturated to 1 10 6 OH
0
I 10 to 5 10 8
Pb 2 10 1 to 1 10 6 Hg 2 ,Ag ,Cu 2
0
Ag /S 2 Ag :10 to 1 10 7 Hg 2
0
S 2 : 10 to 1 10 7
0
SCN 10 to 1 10 6 I ,Br ,CN ,S 2
a
From Orion Guide to Ion Analysis, Orion Research, Cambridge, MA, 1983. With permission.