Page 172 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 172
5-2 ION-SELECTIVE ELECTRODES 157
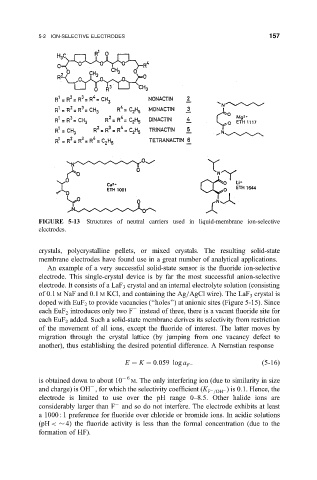
FIGURE 5-13 Structures of neutral carriers used in liquid-membrane ion-selective
electrodes.
crystals, polycrystalline pellets, or mixed crystals. The resulting solid-state
membrane electrodes have found use in a great number of analytical applications.
An example of a very successful solid-state sensor is the ¯uoride ion-selective
electrode. This single-crystal device is by far the most successful anion-selective
electrode. It consists of a LaF 3 crystal and an internal electrolyte solution (consisting
of 0.1 M NaF and 0.1 M KCl, and containing the Ag=AgCl wire). The LaF 3 crystal is
doped with EuF 2 to provide vacancies (``holes'') at anionic sites (Figure 5-15). Since
each EuF 2 introduces only two F instead of three, there is a vacant ¯uoride site for
each EuF 2 added. Such a solid-state membrane derives its selectivity from restriction
of the movement of all ions, except the ¯uoride of interest. The latter moves by
migration through the crystal lattice (by jumping from one vacancy defect to
another), thus establishing the desired potential difference. A Nernstian response
E K 0:059 log a
5-16
F
is obtained down to about 10 6 M. The only interfering ion (due to similarity in size
F =OH
and charge) is OH , for which the selectivity coef®cient (K ) is 0.1. Hence, the
electrode is limited to use over the pH range 0±8.5. Other halide ions are
considerably larger than F and so do not interfere. The electrode exhibits at least
a 1000 : 1 preference for ¯uoride over chloride or bromide ions. In acidic solutions
(pH < 4) the ¯uoride activity is less than the formal concentration (due to the
formation of HF).