Page 168 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 168
5-2 ION-SELECTIVE ELECTRODES 153
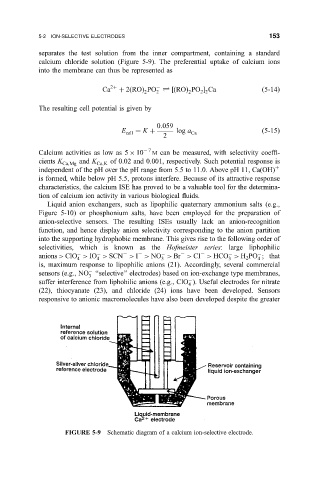
separates the test solution from the inner compartment, containing a standard
calcium chloride solution (Figure 5-9). The preferential uptake of calcium ions
into the membrane can thus be represented as
Ca 2 2
RO PO
RO PO Ca
5-14
2 2 2 2 2
The resulting cell potential is given by
0:059
E K log a
5-15
cell Ca
2
Calcium activities as low as 5 10 7 M can be measured, with selectivity coef®-
cients K Ca;Mg and K Ca;K of 0.02 and 0.001, respectively. Such potential response is
independent of the pH over the pH range from 5.5 to 11.0. Above pH 11, Ca(OH)
is formed, while below pH 5.5, protons interfere. Because of its attractive response
characteristics, the calcium ISE has proved to be a valuable tool for the determina-
tion of calcium ion activity in various biological ¯uids.
Liquid anion exchangers, such as lipophilic quaternary ammonium salts (e.g.,
Figure 5-10) or phosphonium salts, have been employed for the preparation of
anion-selective sensors. The resulting ISEs usually lack an anion-recognition
function, and hence display anion selectivity corresponding to the anion partition
into the supporting hydrophobic membrane. This gives rise to the following order of
selectivities, which is known as the Hofmeister series: large liphophilic
anions > ClO > IO > SCN > I > NO > Br > CI > HCO > H 2 PO ; that
4 4 3 3 4
is, maximum response to lipophilic anions (21). Accordingly, several commercial
sensors (e.g., NO ``selective'' electrodes) based on ion-exchange type membranes,
3
suffer interference from liphohilic anions (e.g., ClO ). Useful electrodes for nitrate
4
(22), thiocyanate (23), and chloride (24) ions have been developed. Sensors
responsive to anionic macromolecules have also been developed despite the greater
FIGURE 5-9 Schematic diagram of a calcium ion-selective electrode.