Page 169 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 169
154 POTENTIOMETRY
FIGURE 5-10 A quaternary alkyl ammonium chloride.
dif®culty in identifying appropriate membrane chemistry that yields a signi®cant and
selective response (25,26). A very successful example is the use of the quaternary
ammonium salt tridodecylmethylammonium chloride (TDMAC) for detecting the
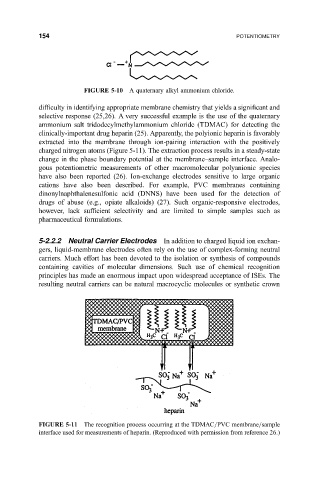
clinically-important drug heparin (25). Apparently, the polyionic heparin is favorably
extracted into the membrane through ion-pairing interaction with the positively
charged nitrogen atoms (Figure 5-11). The extraction process results in a steady-state
change in the phase boundary potential at the membrane±sample interface. Analo-
gous potentiometric measurements of other macromolecular polyanionic species
have also been reported (26). Ion-exchange electrodes sensitive to large organic
cations have also been described. For example, PVC membranes containing
dinonylnaphthalenesulfonic acid (DNNS) have been used for the detection of
drugs of abuse (e.g., opiate alkaloids) (27). Such organic-responsive electrodes,
however, lack suf®cient selectivity and are limited to simple samples such as
pharmaceutical formulations.
5-2.2.2 Neutral Carrier Electrodes In addition to charged liquid ion exchan-
gers, liquid-membrane electrodes often rely on the use of complex-forming neutral
carriers. Much effort has been devoted to the isolation or synthesis of compounds
containing cavities of molecular dimensions. Such use of chemical recognition
principles has made an enormous impact upon widespread acceptance of ISEs. The
resulting neutral carriers can be natural macrocyclic molecules or synthetic crown
FIGURE 5-11 The recognition process occurring at the TDMAC=PVC membrane=sample
interface used for measurements of heparin. (Reproduced with permission from reference 26.)