Page 174 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 174
5-2 ION-SELECTIVE ELECTRODES 159
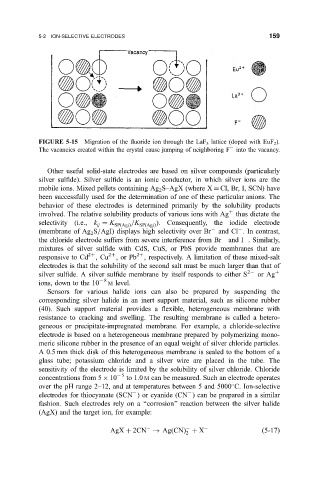
FIGURE 5-15 Migration of the ¯uoride ion through the LaF 3 lattice (doped with EuF 2 ).
The vacancies created within the crystal cause jumping of neighboring F into the vacancy.
Other useful solid-state electrodes are based on silver compounds (particularly
silver sul®de). Silver sul®de is an ionic conductor, in which silver ions are the
mobile ions. Mixed pellets containing Ag 2 S±AgX (where X Cl, Br, I, SCN) have
been successfully used for the determination of one of these particular anions. The
behavior of these electrodes is determined primarily by the solubility products
involved. The relative solubility products of various ions with Ag thus dictate the
selectivity (i.e., k K SP
Agi =K SP
Agj ). Consequently, the iodide electrode
ij
(membrane of Ag 2 S=AgI) displays high selectivity over Br and Cl . In contrast,
the chloride electrode suffers from severe interference from Br and I . Similarly,
mixtures of silver sul®de with CdS, CuS, or PbS provide membranes that are
responsive to Cd 2 ,Cu 2 ,orPb 2 , respectively. A limitation of these mixed-salt
electrodes is that the solubility of the second salt must be much larger than that of
silver sul®de. A silver sul®de membrane by itself responds to either S 2 or Ag
ions, down to the 10 8 M level.
Sensors for various halide ions can also be prepared by suspending the
corresponding silver halide in an inert support material, such as silicone rubber
(40). Such support material provides a ¯exible, heterogeneous membrane with
resistance to cracking and swelling. The resulting membrane is called a hetero-
geneous or precipitate-impregnated membrane. For example, a chloride-selective
electrode is based on a heterogeneous membrane prepared by polymerizing mono-
meric silicone rubber in the presence of an equal weight of silver chloride particles.
A 0.5 mm thick disk of this heterogeneous membrane is sealed to the bottom of a
glass tube; potassium chloride and a silver wire are placed in the tube. The
sensitivity of the electrode is limited by the solubility of silver chloride. Chloride
concentrations from 5 10 5 to 1.0 M can be measured. Such an electrode operates
over the pH range 2±12, and at temperatures between 5 and 5000 C. Ion-selective
electrodes for thiocyanate (SCN ) or cyanide (CN ) can be prepared in a similar
fashion. Such electrodes rely on a ``corrosion'' reaction between the silver halide
(AgX) and the target ion, for example:
AgX 2CN ! Ag
CN X
5-17
2