Page 188 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 188
6-1 ELECTROCHEMICAL BIOSENSORS 173
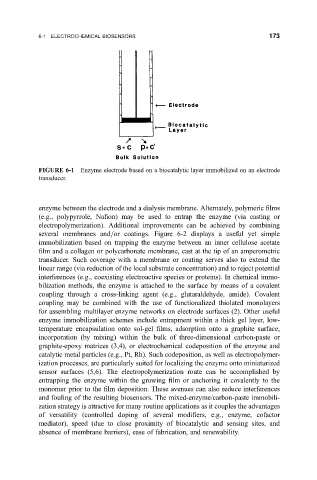
FIGURE 6-1 Enzyme electrode based on a biocatalytic layer immobilized on an electrode
transducer.
enzyme between the electrode and a dialysis membrane. Alternately, polymeric ®lms
(e.g., polypyrrole, Na®on) may be used to entrap the enzyme (via casting or
electropolymerization). Additional improvements can be achieved by combining
several membranes and=or coatings. Figure 6-2 displays a useful yet simple
immobilization based on trapping the enzyme between an inner cellulose acetate
®lm and a collagen or polycarbonate membrane, cast at the tip of an amperometric
transducer. Such coverage with a membrane or coating serves also to extend the
linear range (via reduction of the local substrate concentration) and to reject potential
interferences (e.g., coexisting electroactive species or proteins). In chemical immo-
bilization methods, the enzyme is attached to the surface by means of a covalent
coupling through a cross-linking agent (e.g., glutaraldehyde, amide). Covalent
coupling may be combined with the use of functionalized thiolated monolayers
for assembling multilayer enzyme networks on electrode surfaces (2). Other useful
enzyme immobilization schemes include entrapment within a thick gel layer, low-
temperature encapsulation onto sol-gel ®lms, adsorption onto a graphite surface,
incorporation (by mixing) within the bulk of three-dimensional carbon-paste or
graphite-epoxy matrices (3,4), or electrochemical codeposition of the enzyme and
catalytic metal particles (e.g., Pt, Rh). Such codeposition, as well as electropolymer-
ization processes, are particularly suited for localizing the enzyme onto miniaturized
sensor surfaces (5,6). The electropolymerization route can be accomplished by
entrapping the enzyme within the growing ®lm or anchoring it covalently to the
monomer prior to the ®lm deposition. These avenues can also reduce interferences
and fouling of the resulting biosensors. The mixed-enzyme/carbon-paste immobili-
zation strategy is attractive for many routine applications as it couples the advantages
of versatility (controlled doping of several modi®ers, e.g., enzyme, cofactor
mediator), speed (due to close proximity of biocatalytic and sensing sites, and
absence of membrane barriers), ease of fabrication, and renewability.